从幼年小鼠体内提取的工程细胞外囊泡能增强衰老骨微环境中的线粒体功能并实现年轻化
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
与衰老相关的骨质退化和愈合能力受损仍然是再生医学面临的重大挑战,因此需要创新、高效和有针对性的策略来恢复骨骼健康。在这里,我们设计了源自预处理幼鼠血清的细胞外囊泡 (EV),目的是逆转衰老、增强成骨潜能和提高生物利用率,以恢复衰老骨环境的活力。首先,我们建立了代表不同愈合阶段的骨愈合模型,以确定最有可能改善老年人骨微环境的 EV 类型。其次,我们利用 DSS6 进行骨靶向,以增强所选 EV 在体内的生物效应。在老年小鼠体内,与未经修饰的EVs相比,经过修饰的EVs能有效靶向骨修复部位,更有效地促进骨折愈合。RNA测序发现,线粒体外膜转运酶7(Tomm7)对其基本机制至关重要。沉默Tomm7会明显减弱EVs的积极调节作用。具体来说,工程EVs可通过激活Tomm7介导的Pink1/Parkin有丝分裂途径,增强衰老细胞的线粒体功能,促进衰老骨髓基质细胞(BMSCs)的干性恢复,并逆转衰老骨微环境的不利条件。总之,从幼年小鼠血清中提取的工程EV为治疗骨骼老化提供了另一种方法。所发现的潜在生物学机制为未来精准治疗衰老骨骼提供了有价值的参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
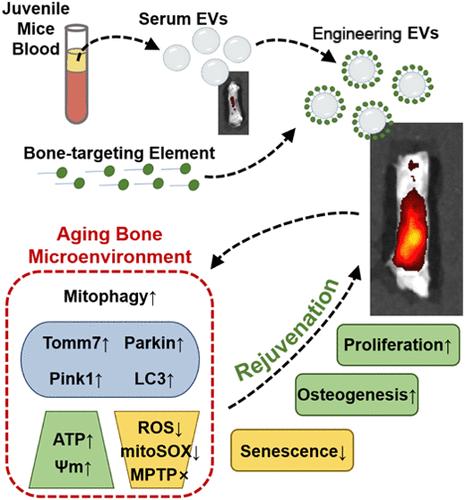
Engineered Extracellular Vesicles Derived from Juvenile Mice Enhance Mitochondrial Function in the Aging Bone Microenvironment and Achieve Rejuvenation
Aging-related bone degeneration and impaired healing capacity remain significant challenges in regenerative medicine, necessitating innovative, efficient, and targeted strategies to restore bone health. Here, we engineered extracellular vesicles (EVs) derived from the serum of pretreated juvenile mice, with the goals of reversing aging, enhancing osteogenic potential, and increasing bioavailability to rejuvenate the aging bone environment. First, we established bone healing models representing different phases of healing to identify the EV type with the highest potential for improving the bone microenvironment in older individuals. Second, we employed DSS6 for bone targeting to enhance the biological effects of the selected EVs in vivo. The engineered EVs effectively targeted bone repair sites and promoted fracture healing more effectively than unmodified EVs in older mice. RNA sequencing revealed that the translocase of outer mitochondrial membrane 7 (Tomm7) is crucial for the underlying mechanism. Silencing Tomm7 significantly diminished the positive regulatory effects of the EVs. Specifically, the engineered EVs may enhance mitochondrial function in aging cells by activating the Tomm7-mediated Pink1/Parkin mitophagy pathway, promoting stemness recovery in aging bone marrow stromal cells (BMSCs) and reversing the adverse conditions of the aging bone microenvironment. Overall, the developed engineered EVs derived from serum from juvenile mice offer an alternative approach for treating aging bones. The identified underlying biological mechanisms provide a valuable reference for precision treatment of aging bones in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


