功能性非血红素二铁(II)配合物催化亚硝酸盐直接还原为一氧化氮,与二铁蛋白 YtfE 有关
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
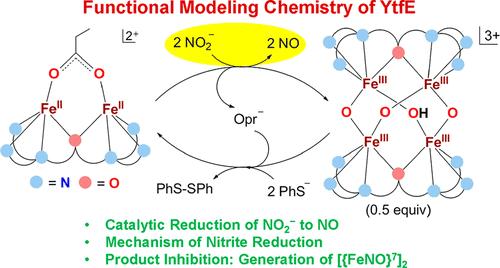
本工作报道了YtfE的功能建模化学,它具有非血红素双铁活性位点,并介导NO2 -直接还原为NO。模型配合物[Fe2(HPTP)Cl2]1+(1)在12 h内以100%的产率将NO2 -还原为NO,生成[Fe4(HPTP)2(μ-O)3(μ-OH)]3+(2)。与YtfE类似,该反应涉及两个Fe(II)中心的逐步氧化和产物(NO)抑制,后者生成[Fe2(HPTP)(NO)2Cl2]1+(3)。配合物3也可以通过[Fe2(HPTP)(NO)2(ClO4)]2+(4)和氯化物的反应合成。配合物1在ph -存在下催化NO2 -还原为NO,但由于形成不溶产物[Fe2(HPTP)(μ-SPh)Cl2](5),其TON值较低。另一种模式配合物[Fe2(HPTP)(OPr)]1+(6),在24 h后以80%的收率将NO2 -还原为NO,生成[Fe2(HPTP)(OPr)(NO)2]1+ (7), TON值为19。第三种模型配合物[Fe2(HPTP)(ClO4)2]1+(8)可将NO2 -还原为NO,但仅在48 h后才能达到100%的产率。比较这些结果表明,Fe(II)中心易于氧化,Fe(II)中心易于与NO2 -配合,并且原位生成的二硝基二铁配合物易于释放NO,从而提高了YtfE功能模型配合物的效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Functional Nonheme Diiron(II) Complexes Catalyze the Direct Reduction of Nitrite to Nitric Oxide in Relevance to the Diiron Protein YtfE
The present work reports the functional modeling chemistry of YtfE, which features a nonheme diiron active site and mediates the direct reduction of NO2– to NO. The model complex, [Fe2(HPTP)Cl2]1+ (1), reduces NO2– to NO in a 100% yield within 12 h and generates [Fe4(HPTP)2(μ-O)3(μ-OH)]3+ (2). Similar to YtfE, the reaction involves stepwise oxidation of two Fe(II) centers and product (NO) inhibition, of which the latter produces [Fe2(HPTP)(NO)2Cl2]1+ (3). Complex 3 could also be synthesized by the reaction of [Fe2(HPTP)(NO)2(ClO4)]2+ (4) and chloride. Complex 1 catalyzes the reduction of NO2– to NO in the presence of PhS–, albeit with a low TON of 5, due to the formation of an insoluble product, [Fe2(HPTP)(μ-SPh)Cl2] (5). Another model complex [Fe2(HPTP)(OPr)]1+ (6), reduced NO2– to NO in an 80% yield after 24 h, generated [Fe2(HPTP)(OPr)(NO)2]1+ (7), and offered a TON of 19. The third model complex, [Fe2(HPTP)(ClO4)2]1+ (8), could reduce NO2– to NO in a 100% yield but only after 48 h. A comparison of these results establishes that easy oxidation of the Fe(II) centers, easy accessibility of the Fe(II) centers for the coordination of NO2–, and easy release of NO from the in situ generated dinitrosyl diiron complex increase the efficiency of the functional model complexes of YtfE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


