靶向双微滴调节破骨细胞分化和功能:对抗骨质疏松症的新治疗方法
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
骨质疏松症是一种以骨量减少和结构恶化为特征的疾病,它仍然是一个主要的公共卫生问题,特别是在全球人口老龄化的背景下。过度的破骨细胞形成是骨质疏松症的标志。活化t细胞胞质1的转录因子核因子(NFATc1)在破骨细胞的早期分化中必不可少,协调必需基因的表达,而在后期,组织蛋白酶K (CTSK)对成熟破骨细胞的骨吸收活性至关重要。在这里,我们通过靶向NFATc1和CTSK,通过调节破骨细胞分化的早期阶段和成熟破骨细胞的功能来制造超声响应微滴(MDs)。在人骨髓间充质基质细胞(hBMSCs)和小鼠RAW 264.7巨噬细胞中评估了这些双MDs的内化,并进行了生物相容性试验。在体外进一步研究其对成骨和破骨细胞的影响,并在体内对骨质疏松大鼠模型进行分析。双MDs表现出明确的核壳结构,并表现出有效的细胞摄取和最小的细胞毒性。此外,双MDs对hBMSCs成骨分化的影响很小。在体外破骨细胞生成实验中,双MDs通过协同抑制作用有效抑制破骨细胞的分化和形成。体内研究表明,接受双MDs的骨质疏松大鼠对卵巢切除术引起的骨质流失具有显著的保护作用。这些结果突出了双MDs作为一种复杂的、有针对性的骨质疏松治疗方法的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
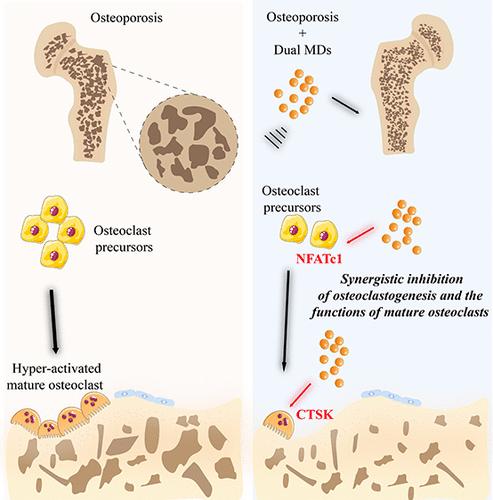
Targeted Dual Microdroplets for Modulating Osteoclast Differentiation and Function: A Novel Therapeutic Approach to Combat Osteoporosis
Osteoporosis, a condition marked by reduced bone mass and structural deterioration, continues to be a major public health concern, especially as global populations age. Excessive osteoclast formation is a hallmark of osteoporosis. The transcription factor nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) is indispensable for the early differentiation of osteoclasts, orchestrating the expression of essential genes, while at the later stages, cathepsin K (CTSK) is essential for bone resorption activities of mature osteoclasts. Here, we fabricated ultrasound-responsive microdroplets (MDs) by modulating both the early stages of osteoclast differentiation and the functions of mature osteoclasts via targeting the NFATc1 and CTSK. The internalization of these dual MDs was evaluated in human bone marrow-derived mesenchymal stromal cells (hBMSCs) and murine RAW 264.7 macrophages, alongside the biocompatibility assay. Their effects on osteogenesis and osteoclastogenesis were further investigated in vitro, followed by in vivo analysis in osteoporotic rat models. The dual MDs exhibited a well-defined core–shell structure and demonstrated efficient cellular uptake with minimal cytotoxicity. Furthermore, dual MDs showed a minimal effect on the osteogenic differentiation of the hBMSCs. In in vitro osteoclastogenesis assays, dual MDs effectively suppressed both osteoclast differentiation and formation through a synergistic inhibitory effect. In vivo studies demonstrated that osteoporotic rats receiving dual MDs showed significant protection against bone loss induced by ovariectomy. These results highlight the potential of dual MDs as a sophisticated, targeted therapeutic approach to osteoporosis treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


