氧增强Au1Agx/SiO2催化剂对CO氧化的催化效率
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过不同的大气工艺制备和预处理了一系列二氧化硅支撑的 Au1Agx 合金(x = 0、0.2、0.3、1.0 和 3.0)。利用 X 射线衍射 (XRD)、X 射线光电子能谱 (XPS)、紫外-可见 (UV-vis) 光谱和透射电子显微镜 (TEM) 对这些材料的物理化学特性进行了系统表征。研究结果表明,氧参与预处理使氧物种掺杂到合金结构中,从而导致晶格膨胀,并显著提高了催化活性。通过连续的还原和氧化预处理,Au1Ag0.3/SiO2 的催化活性得到了提高,100% CO 转化温度降低了约 500 K。密度泛函理论(DFT)计算表明,引入的氧物种很可能存在于 AuAg 合金的次表层,并参与反应或改变表面 Ag 的电子结构,从而提高 CO 氧化的催化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
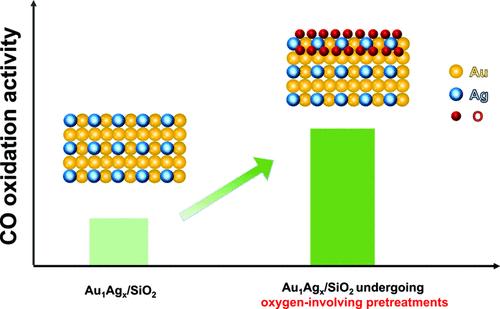
Oxygen Species Enhanced Catalytic Efficiency of Au1Agx/SiO2 Catalysts for CO Oxidation
A series of Au1Agx alloys (x = 0, 0.2, 0.3, 1.0, and 3.0) supported over SiO2 has been prepared and pretreated via different atmospheric processes. The physical–chemical properties of these materials have been systematically characterized using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), ultraviolet–visible (UV–vis) spectroscopy, and transmission electron microscopy (TEM). The results reveal that oxygen species are doped into the alloy structure by the oxygen-involved pretreatment, leading to lattice expansion as well as a significant increase in catalytic activity. Improvement in the catalytic activity of Au1Ag0.3/SiO2 through sequential reduction and oxidation pretreatment was evidenced by a decrease in the temperature of 100% CO conversion by approximately 500 K. A volcano trend in catalytic activity is found as the Ag composition is increased in the alloy structure. Density-functional theory (DFT) calculations suggest that the introduced oxygen species are likely present at the subsurface of the AuAg alloy and involved in the reaction or in modifying the electronic structure of surface Ag, thereby enhancing the catalytic activity for CO oxidation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


