用于CRISPR-Cas9核糖核蛋白体内肺递送的脂质纳米颗粒允许临床靶标的基因编辑
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
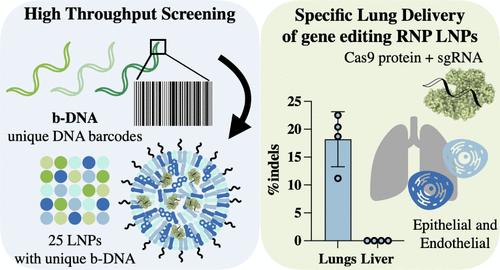
在过去的10年里,CRISPR-Cas9以其模块化、简单性和有效性彻底改变了基因编辑领域。它已被应用于体内模型的创建,以进一步了解人类生物学,并对遗传疾病的治疗。然而,CRISPR-Cas9在临床上的应用仍然存在明显的递送障碍,特别是在体内和肝外应用方面。在这项工作中,高通量分子条形码技术与传统筛选方法一起用于同时评估包裹核糖核蛋白(RNPs)的LNP配方的体外基因编辑效率和体内生物分布。这导致鉴定出一种亲肺LNP制剂,该制剂在肺内皮细胞和上皮细胞中显示出有效的基因编辑,靶向模型报告细胞和临床相关的基因组靶点。此外,这种LNP在肝脏中没有脱靶indel形成,使其成为肺编辑应用的高度特异性肝外递送系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lipid Nanoparticles for In Vivo Lung Delivery of CRISPR-Cas9 Ribonucleoproteins Allow Gene Editing of Clinical Targets
In the past 10 years, CRISPR-Cas9 has revolutionized the gene-editing field due to its modularity, simplicity, and efficacy. It has been applied for the creation of in vivo models, to further understand human biology, and toward the curing of genetic diseases. However, there remain significant delivery barriers for CRISPR-Cas9 application in the clinic, especially for in vivo and extrahepatic applications. In this work, high-throughput molecular barcoding techniques were used alongside traditional screening methodologies to simultaneously evaluate LNP formulations encapsulating ribonucleoproteins (RNPs) for in vitro gene-editing efficiency and in vivo biodistribution. This resulted in the identification of a lung-tropic LNP formulation, which shows efficient gene editing in endothelial and epithelial cells within the lung, targeting both model reporter and clinically relevant genomic targets. Further, this LNP shows no off-target indel formation in the liver, making it a highly specific extrahepatic delivery system for lung-editing applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


