肠道微生物源性d-(+)-苹果酸通过p-p38/ATF1信号通路促进pBD1表达,维持猪肠道健康
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
本研究旨在鉴定增强宿主防御肽(host defense peptides, hdp)表达的营养物质,并评价其应用效果和调控机制,从而推进对宿主防御肽营养调控的探索。为了实现这一目标,我们利用IPEC-1和IPEC-J2细胞构建了16株稳定的荧光报告猪上皮细胞系,该细胞系由靶向8个猪肠HDPs基因的启动子驱动。通过非靶向代谢组学测序和高通量筛选,15种代谢物被鉴定为pBD1表达的潜在增强物,其中d-(+)-苹果酸(DMA)被认为是最有效的候选物。转录组学和western blot分析表明,DMA主要通过p-p38/ATF1信号通路增强pBD1的表达。功能研究表明,DMA可显著改善肠道屏障完整性,减轻肠道损伤。总的来说,这项工作成功地建立了启动子驱动的荧光报告细胞系,并鉴定了微生物来源的代谢产物,如DMA,增强pBD1表达,作为维持猪肠道健康的有希望的抗生素替代品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
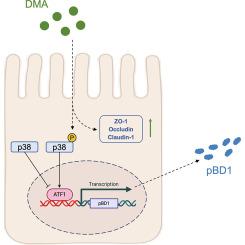
Intestinal microbiota-derived d-(+)-malic acid promotes pBD1 expression via p-p38/ATF1 signaling pathway to maintain porcine intestinal health
This study aims to identify nutrients that enhance the expression of host defense peptides (HDPs) and to evaluate their application effects and regulatory mechanisms, thereby advancing the exploring of nutritional regulation of HDPs. To achieve this, we constructed 16 stable fluorescent reporter porcine epithelial cell lines, driven by promoters targeting eight porcine intestinal HDPs genes, using IPEC-1 and IPEC-J2 cells. Through untargeted metabolomics sequencing and high-throughput screening, 15 metabolites were identified as potential enhancers of pBD1 expression, with d-(+)-malic acid (DMA) emerged as the most effective candidate. Transcriptomic and western blot analysis suggested that DMA enhances pBD1 expression primarily via the p-p38/ATF1 signaling pathways. Functional studies demonstrated that DMA significantly improved intestinal barrier integrity and alleviated intestinal damage. Overall, this work successfully established promoter-driven fluorescent reporter cell lines and identified microbiota-derived metabolites enhancing pBD1 expression, such as DMA, as promising alternatives to antibiotics for maintaining porcine intestinal health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


