酶诱变和纳米复合材料集成增强尿酸生物传感器稳定性
IF 8.5
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2025-04-03
DOI:10.1016/j.ijbiomac.2025.142826
引用次数: 0
摘要
尿酸氧化酶(UOx)具有开发尿酸传感器的潜力,但其低催化效率和环境脆弱性限制了其广泛应用。本研究采用半合理设计方法对mojavensis XH1 UOx进行工程改造,获得了催化效率提高(比活性提高2.84倍)、热稳定性提高(熔融温度变化+7.54 °C)、操作稳定性提高(半衰期延长1.94倍)的突变体UOxQ170K。结构分析表明,Q170K突变通过优化的氢键和疏水相互作用稳定了底物结合,从而在保持催化可及性的同时降低了构象灵活性。为了利用这些改进,我们整合了用于酶固定的沸石咪唑酸框架-8 (ZIF-8)、用于增强电子转移的碳纳米管(CNTs)和用于H2O2信号放大的辣根过氧化物酶(HRP),以合成多功能HRP@ZIF-8/CNT-UOxQ170K纳米杂化物。该设计最小化了中间扩散距离,放大了电化学响应,在玻碳电极上实现了UA的检出限为0.031 μM,灵敏度为32.26 μA μM−1 cm−2。该传感器对常见代谢物表现出强大的抗干扰能力,在14 天内保持了>; 85% %的信号稳定性。这项工作建立了一种将酶工程与纳米材料设计相结合的协同方法,以推进电化学生物传感平台,为开发具有放大信号转导的强大酶检测系统提供了范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
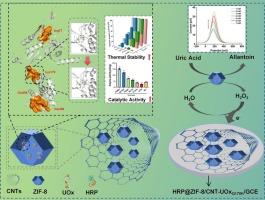
Stability enhancement of uric acid biosensor via enzyme mutagenesis and nanocomposite integration
Urate oxidase (UOx) shows potential for developing uric acid (UA) sensors, but its low catalytic efficiency and environmental fragility limit broader application. This study engineered Bacillus mojavensis XH1 UOx using a semi-rational design approach, resulting in mutant UOxQ170K with enhanced catalytic efficiency (2.84-fold increase in specific activity), thermal stability (melting temperature change +7.54 °C), and operational stability (1.94-fold extension in half-life). Structural analysis revealed that the Q170K mutation stabilized substrate binding via optimized hydrogen bonding and hydrophobic interactions, thereby reducing conformational flexibility while maintaining catalytic accessibility. To leverage these improvements, we have integrated zeolitic imidazolate framework-8 (ZIF-8) for enzyme immobilization, carbon nanotubes (CNTs) for enhanced electron transfer, and horseradish peroxidase (HRP) for H2O2 signal amplification to synthesise a multifunctional HRP@ZIF-8/CNT-UOxQ170K nanohybrid. This design minimized intermediate diffusion distances and amplified electrochemical responses, achieving a limit of detection of 0.031 μM and sensitivity of 32.26 μA μM−1 cm−2 for UA on a glassy carbon electrode. The sensor exhibited robust anti-interference capability against common metabolites and retained >85 % signal stability over 14 days. This work establishes a synergistic approach combining enzyme engineering with nanomaterial design to advance electrochemical biosensing platforms, providing a paradigm for developing robust enzymatic detection systems with amplified signal transduction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


