易于处理的NaBArF4催化芳基胺C(sp2) -H键的选择性亲电氘化方法
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
氘化芳基胺在医药领域,特别是在氘化药物的开发中越来越受到追捧。传统的合成方法通常涉及苛刻的条件或预先制备的试剂。本研究介绍了一种温和的、无金属的芳基胺选择性氘化方法,利用氧化氘(D2O)和市售催化剂,四(3,5-二(三氟甲基)苯基)硼酸盐(NaBArF4)。该反应在温和的条件下进行,并且与广泛的底物兼容,包括敏感的官能团。机制研究强调了非配位Na+在催化中的关键作用,强调了NaBArF4和弱配位阴离子(WCAs)在合成化学中的广泛潜力。该方法为氘化芳胺的合成提供了一种高效、可持续的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
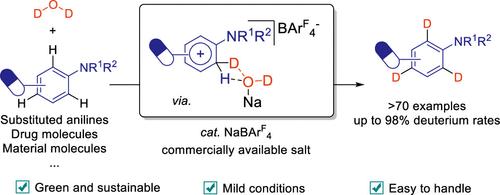
Easily Handled NaBArF4 Catalyzed Selective Electrophilic Deuteration Method for C(sp2)–H Bond of Aryl Amines
Deuterated aryl amines are increasingly sought after in pharmaceuticals, particularly in the development of deuterated drugs. Traditional methods for their synthesis often involve harsh conditions or preprepared reagents. This study introduces a mild, metal-free method for the selective deuteration of aryl amines, utilizing deuterium oxide (D2O) and a commercially available catalyst, tetrakis(3,5-bis(trifluoromethyl)phenyl)borate (NaBArF4). The reaction is performed under moderate conditions and is compatible with a wide range of substrates, including sensitive functional groups. Mechanistic studies highlight the crucial role of noncoordinated Na+ in catalysis, underscoring the broader potential of NaBArF4 and weakly coordinating anions (WCAs) in synthetic chemistry. This method offers an efficient and sustainable approach to synthesizing deuterated aryl amines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


