双咪唑基聚离子液体功能化烃类高效吸附甲基橙和2,4-二氯苯氧乙酸钠
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
环保型水煤浆在处理阳离子污染物方面表现出色,但其处理阴离子的能力有限。阳离子咪唑离子液体带有正电荷,对水炭表面进行改性可增加其正电荷,从而提高其去除阴离子的能力。本文利用竹粉和 N,N'-亚甲基双(1-(3-乙烯基咪唑))氯通过自由基聚合反应进行水热碳化得到的水炭,制备了双咪唑基聚(离子液体)功能化水炭(BIPIL-HC),并使用不同的仪器对其进行了表征。通过批次吸附实验,包括初始浓度和温度、溶液 pH 值、接触时间对吸附的影响以及再生实验,研究了 BIPIL-HC 对甲基橙(MO)和 2,4-D Na(2,4-二氯苯氧乙酸钠)的吸附行为。吸附动力学和等温线符合伪二阶动力学和 Langmuir 模型。BIPIL-HC 对 MO 和 2,4-D Na 的吸附容量分别达到 554.91 和 565.50 mg-g-1。在不同的 pH 值条件下,BIPIL-HC 也能有效去除 MO 和 2,4-D Na,而且可重复使用。机理分析表明,氢键、离子交换、静电和 π-π 相互作用促进了 BIPIL-HC 对这两种污染物的吸附。尤其是 BIPIL-HC 中的咪唑基团通过阴离子交换和静电作用对这两种阴离子污染物的捕获能力起着决定性作用。这些结果证实,利用离子液体功能化作为一种改性水炭的方法,可有效提高水炭对阴离子废水的处理能力,所获得的 BIPIL-HC 在阴离子废水处理方面具有广阔的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
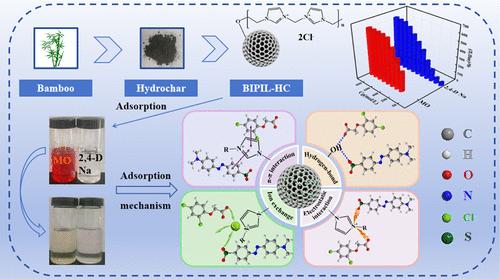
Bis-Imidazolium-Based Poly(Ionic Liquid)-Functionalized Hydrochar for Efficient Sorption of Methyl Orange and Sodium 2,4-Dichlorophenoxyacetate
Environmentally friendly hydrochar demonstrates excellent performance in the treatment of cationic pollutants, yet its capability to address anions is limited. Cationic imidazolium ionic liquids carry a positive charge, and modification of the surface of hydrochar can increase its positive charge, thereby improving its ability to remove anions. Herein, bis-imidazolium-based poly(ionic liquid)-functionalized hydrochar (BIPIL-HC) was prepared using hydrochar derived from the hydrothermal carbonization of bamboo powder and N,N’-methylene-bis(1-(3-vinylimidazolium)) chloride via free radical polymerization and characterized using different instruments. The behavior of BIPIL-HC in adsorbing methyl orange (MO) and sodium 2,4-dichlorophenoxyacetate (2,4-D Na) was studied by using batch adsorption experiments, including the effects of initial concentration and temperature, solution pH, contact time on adsorption, and regeneration experiments. The adsorption kinetics and isotherms conformed to pseudo-second-order kinetics and Langmuir models. The adsorbing capacity of BIPIL-HC for MO and 2,4-D Na reached 554.91 and 565.50 mg·g–1, respectively. BIPIL-HC is also effective in removing MO and 2,4-D Na under diverse pH values and is highly reusable. Mechanism analysis shows that hydrogen bonding, ion exchange, electrostatic, and π-π interactions promote the adsorption of the two pollutants by BIPIL-HC. Particularly, the imidazolium group of BIPIL-HC is decisive in its capture ability of the two anionic pollutants through anion exchange and electrostatic interaction. These results confirm that the use of ionic liquid functionalization as a method for modifying hydrochar can effectively enhance the treatment capacity of hydrochar for anionic wastewater, and the obtained BIPIL-HC shows promising value in anionic wastewater handling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


