热化学非均相催化的无线电位测定
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
催化剂的电化学电位定义了液体介质中催化的自由能分布,对于支撑在导电材料上或连接到外部电路的催化剂,电化学电位很容易测量。然而,在电绝缘体上支持的热化学催化剂的潜力很难量化,从而阻碍了对热化学催化过程中电化学极化作用的统一理解。在这里,我们开发了一种方法,通过引入低浓度的氧化还原活性分子,在催化剂和传感电极之间建立无线电连接,来量化支撑在绝缘体上的金属催化剂的电化学电位。使用这种方法与同步速率测量相结合,我们证明了甲酸在sio2负载和al2o3负载与tio2负载Pt上氧化脱氢的不同速率电位标度,并找到了特定于sio2负载Pt的失活模式。这些发展使电化学极化在热化学催化中的作用得到全面研究,并补充了现有的催化机理研究工具包。本文章由计算机程序翻译,如有差异,请以英文原文为准。
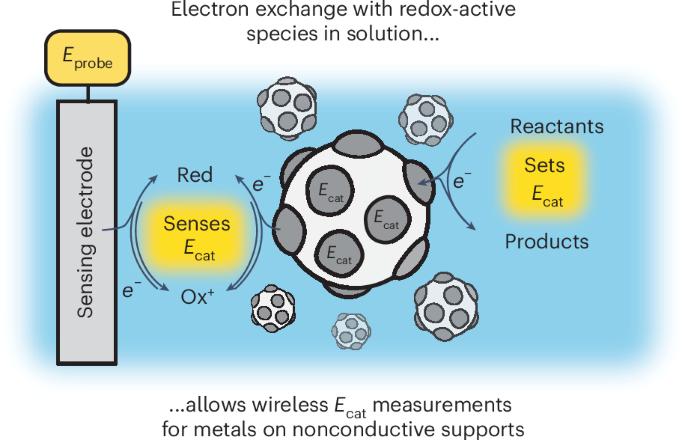

Wireless potentiometry of thermochemical heterogeneous catalysis
The electrochemical potential of a catalyst defines the free-energy landscape of catalysis in liquid media and is readily measured for catalysts supported on conductive materials or wired to external circuits. However, the potential is difficult to quantify for thermochemical catalysts supported on electrical insulators, thereby impeding a unifying understanding of the role of electrochemical polarization during thermochemical catalysis. Here we develop a methodology to quantify the electrochemical potential of metal catalysts supported on insulators by introducing low concentrations of redox-active molecules that establish wireless electrical connections between the catalyst and a sensing electrode. Using this approach in tandem with simultaneous rate measurements, we demonstrate distinct rate-potential scalings for oxidative dehydrogenation of formic acid on SiO2-supported and Al2O3-supported versus TiO2-supported Pt and find deactivation modes specific to SiO2-supported Pt. These developments enable the comprehensive investigation of the role of electrochemical polarization in thermochemical catalysis and complement the existing toolkit for mechanistic investigation in catalysis. Interfacial polarization influences catalytic reactions occurring at solid–liquid interfaces, but its measurement was previously limited to conductive materials. Now redox-active molecules enable electrochemical potential measurements of metal catalysts, even on insulating supports.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


