M2巨噬细胞膜包裹的骨髓间充质干细胞外泌体抑制关节假体周围炎症
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
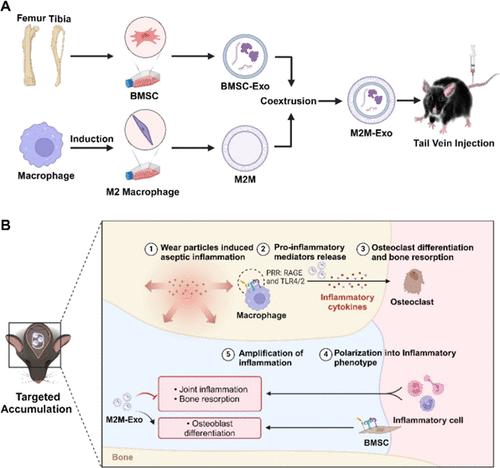
假体周围骨溶解(PPOL)是全关节置换术后的严重并发症,探索这种并发症的治疗方法具有重要的社会意义。来源于骨髓间充质干细胞的外泌体(BMSC-Exos, Exos)具有多种细胞功能,如抑制破骨细胞形成、抑制炎症进展、促进M2巨噬细胞极化等。然而,独立外泌体容易被免疫系统识别和吞噬,半衰期短,缺乏特异性。本研究基于M2巨噬细胞在多种因素的调控下所具有的归巢效应。将其与细胞膜封装技术相结合,将BMSC-Exos包埋在M2巨噬细胞的膜内(M2M-Exos),目的是抑制炎症,治疗PPOL。发现M2M-Exos可以靶向PPOL区域,增强BMSC-Exos的治疗效果,减少磨损颗粒引起的颅骨骨溶解。此外,M2M-Exos通过细胞膜提供免疫伪装,使BMSC-Exos逃避体内单核巨噬细胞系统的清除。因此,本研究证明了M2M-Exos的靶向能力及其在预防PPOL中的独特作用。这些仿生纳米颗粒为PPOL治疗建立了靶向纳米药物递送系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Encapsulated in M2 Macrophage Cell Membrane Targeted to Inhibit Joint Periprosthetic Inflammation
Periprosthetic osteolysis (PPOL) is a serious complication following total joint replacement surgery, and exploring treatments for this complication is of significant societal importance. Exosomes derived from bone marrow mesenchymal stem cells (BMSC-Exos, Exos) have diverse cellular functions, such as inhibiting osteoclast formation, suppressing inflammation progression, and promoting M2 macrophage polarization. However, standalone Exosomes are easily recognized and phagocytosed by the immune system, have a short half-life, and lack specificity. This study is based on the homing effect possessed by M2 macrophages under the regulation of various factors. By combining this with cell membrane encapsulation technology and embedding BMSC-Exos within the membrane of M2 macrophages (M2M-Exos), the aim is to inhibit inflammation and treat PPOL. It was found that M2M-Exos can target the PPOL area, enhancing the therapeutic effects of the BMSC-Exos and reducing wear particle-induced cranial osteolysis. Additionally, M2M-Exos provide immune camouflage through the cell membrane, allowing the BMSC-Exos to evade clearance by the mononuclear macrophage system in the body. Therefore, the study demonstrates the targeting ability of M2M-Exos and their unique role in preventing PPOL. These biomimetic nanoparticles establish a targeted nanodrug delivery system for PPOL treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


