甲酸酯的脱羧和脱氢偶联:从甲酸乙酯到高附加值二胺。
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从醇的脱氧碳碳键形成仍然是一个艰巨的挑战,以更清洁的偶联反应。在这项工作中,我们首先利用预先引入的甲酰基的双重作用,解锁了一种新的苯甲醇的均偶联升级模式,甲酰基既可以促进更容易的C-O键解离,又可以作为内源性氢源。作为概念验证,采用Ru/MoS2催化剂实现了甲酸乙酯对1,2-二(4-甲氧基苯基)乙烷的脱羧和脱氢均偶联,优化产率为66%。结构表征和控制实验表明,Ru和Mo之间存在协同效应。进一步设计了定制化的硝化还原路线,将偶联产物升级为高附加值的芳香族二胺,以甲酸茴香酯为原料,总收率达到56%。该方法为醇通过酯中间体催化转化开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
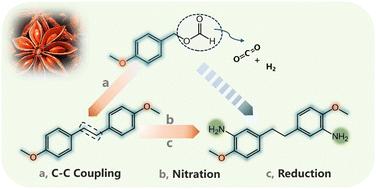
The decarboxylative and dehydrogenative coupling of formate: from anisyl formate to a high value-added diamine†
The deoxygenative carbon–carbon bond formation from alcohols remains a formidable challenge toward cleaner coupling reactions. In this work, we first unlocked a novel homocoupling upgrading mode of benzyl alcohol by virtue of the two-side roles of the pre-introduced formyl group, which serves as both impetus for easier C–O bond dissociation and an endogenous hydrogen source. As a proof-of-concept, the decarboxylative and dehydrogenative homocoupling of anisyl formate toward 1,2-bis(4-methoxyphenyl)ethane was realized using a Ru/MoS2 catalyst with an optimized yield of 66%. Structural characterizations and control experiments indicated the synergy effect between Ru and Mo. A tailored nitration–reduction route was further designed for upgrading the coupling product into a high value-added aromatic diamine, the overall yield of which reached 56% based on anisyl formate. This method opens a new avenue for catalytic transformations of alcohols through ester intermediates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


