矿物表面特异性纳米塑性吸附:来自石英晶体微平衡实验和分子模拟模拟的见解
IF 8
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
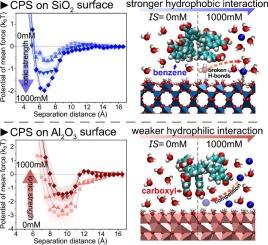
纳米塑料(NP)在土壤和天然水中的迁移主要受矿物表面吸附的控制,而长期静电相互作用通常被认为是主要的作用力。本文主要研究了疏水和亲水性相互作用在纳米塑料吸附中的作用。在与环境相关的离子强度条件下,我们进行了石英晶体微天平(QCM)沉积实验和基于分子动力学(MD)的平均力势(PMF)计算,研究了羧化聚苯乙烯(CPS) NPs在SiO2和Al2O3表面的吸附。QCM测量表明,离子强度的增加增强了NP在SiO2上的沉积,但降低了在Al2O3上的沉积。原子PMF计算证实了这些结果,发现随着离子强度的增加,CPS-NP在SiO2上的负自由能增加,而在Al2O3上的正自由能增加。与传统的DLVO理论相比,我们的MD模拟预测了恒定的stern层厚度,与离子强度无关,并证明了CPS-NP通过疏水性苯基吸附SiO2,通过亲水性羧基吸附Al2O3。较高的电解质浓度通过破坏界面水结构增强了SiO2上的疏水相互作用,而积累的离子阻碍了Al2O3上NP的沉积。这些发现强调了疏水和亲水性相互作用在np -矿物系统中的关键作用,这在预测np的环境运输中经常被忽视。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mineral surface-specific nanoplastic adsorption: Insights from quartz crystal microbalance experiment and molecular modeling simulations
Nanoplastic (NP) transport in soil and natural water is primarily controlled by adsorption onto mineral surfaces, with long-range electrostatic interactions traditionally considered the main force. This study focuses on the role of hydrophobic and hydrophilic interactions in the nanoplastic adsorption. We performed quartz crystal microbalance (QCM) deposition experiments and molecular dynamics (MD)-based potential of mean force (PMF) calculations for the adsorption of carboxylated polystyrene (CPS) NPs on SiO2 and Al2O3 surfaces under environmentally relevant ionic strength conditions. QCM measurements showed that increasing ionic strength enhanced NP deposition on SiO2 but reduced it on Al2O3. Atomistic PMF calculations corroborated these results, revealing more negative free energy of CPS-NP adsorption on SiO2 and more positive on Al2O3 with increasing ionic strength. Contrasting with traditional DLVO theory, our MD simulations predicted a constant Stern-layer thickness independent of ionic strengths and demonstrated CPS-NP adsorption to SiO2 via hydrophobic benzene groups and to Al2O3 via hydrophilic carboxyl groups. Higher electrolyte concentrations strengthened hydrophobic interactions on SiO2 by disrupting interfacial water structure, while accumulated ions hindered NP deposition on Al2O3. These findings highlight the critical role of hydrophobic and hydrophilic interactions in NP–mineral systems, which is often neglected in predicting the environmental transport of NPs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science of the Total Environment
环境科学-环境科学
CiteScore
17.60
自引率
10.20%
发文量
8726
审稿时长
2.4 months
期刊介绍:
The Science of the Total Environment is an international journal dedicated to scientific research on the environment and its interaction with humanity. It covers a wide range of disciplines and seeks to publish innovative, hypothesis-driven, and impactful research that explores the entire environment, including the atmosphere, lithosphere, hydrosphere, biosphere, and anthroposphere.
The journal's updated Aims & Scope emphasizes the importance of interdisciplinary environmental research with broad impact. Priority is given to studies that advance fundamental understanding and explore the interconnectedness of multiple environmental spheres. Field studies are preferred, while laboratory experiments must demonstrate significant methodological advancements or mechanistic insights with direct relevance to the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


