羧壳蛋白CcmK2组装成单分散和ph可逆的微粒
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
合成纳米和微粒已成为生物技术的重要工具。与合成颗粒相比,基于蛋白质的隔室具有明显的优势,例如生物可降解性和生物相容性,但它们的发展仍处于起步阶段。细菌微室(BMCs)是一种基于蛋白质的细胞器,由包裹酶核的蛋白质外壳组成。bmc具有自组装、选择性渗透和模块化的特性,使其成为开发生物技术蛋白质隔室的理想候选材料。事实上,一些研究小组已经将BMC外壳和单个外壳蛋白设计成合成纳米反应器和功能化分子支架。扩展BMC壳蛋白组装的各种结构将增加其作为生物技术构建块的多功能性。在这里,我们开发了一种仅使用CcmK2 (β-羧基体BMC的主要六聚体外壳蛋白)体外组装单组分单分散微颗粒的方法。我们报道了一种单一类型的BMC壳蛋白控制组装成固体微粒。高分辨率成像显示CcmK2粒子是径向聚集的纳米管的集合。通过生化表征,确定了可逆组装和残基介导组装的条件。我们发现pH值是最终颗粒大小和分解的关键调节因子。我们的研究将CcmK2颗粒定位为精确控制和自组装的单分散固体蛋白质颗粒,用于未来的生物技术应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
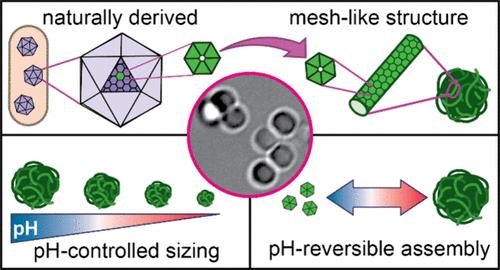
Carboxysome Shell Protein CcmK2 Assembles into Monodisperse and pH-Reversible Microparticles
Synthetic nano- and microparticles have become essential tools in biotechnology. Protein-based compartments offer distinct advantages over synthetic particles, such as biodegradability and biocompatibility, but their development is still in its infancy. Bacterial microcompartments (BMCs) are protein-based organelles consisting of a protein shell encapsulating an enzymatic core. BMCs are self-assembling, selectively permeable, and modular, making them ideal candidates for the development of protein compartments for biotechnology. Indeed, several groups have engineered BMC shells and individual shell proteins into synthetic nanoreactors and functionalized molecular scaffolds. Expanding the variety of architectures assembled from BMC shell proteins will increase their versatility as building blocks in biotechnology. Here, we developed a method for the in vitro assembly of single-component monodisperse microparticles using only CcmK2, the major hexameric shell protein of the β-carboxysome BMC. We report the controlled assembly of a single type of BMC shell protein into a solid microparticle. High-resolution imaging revealed CcmK2 particles to be assemblies of radially clustered nanotubes. Through biochemical characterization, we determined the conditions for reversible assembly and residues mediating assembly. We found that pH is a key regulator of final particle size and disassembly. Our study situates CcmK2 particles as precisely controlled and self-assembling monodisperse solid protein particles for future applications in biotechnology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


