更正“针对宿主网状内皮系统中组织驻留利什曼原虫的Trypanothione还原酶:一种柔性水溶性二茂铁喹啉基临床前候选药物”
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
作者注意到图2A和图6A(ⅰ期解毒酶)和图6B(ⅱ期解毒酶)中的GAPDH面板是相同的,这导致我们在修改后的图6A和图6B中对GAPDH进行了校正。图中的错误是在电子传输原始图像文件时无意中发生的,原始图像文件放置在最终的图6A和6B中。这个错误的修正并不影响结果或结论,因为使用图中所示的渐变色标度(密度测量)创建的热图确实是基于原始的GAPDH(图6A和6B)。本文中公布的其他数据均正确。补充文件中包含的支持数据(表S6)也将保持不变,因为使用原始GAPDH进行计算和统计分析。图6。CQFC1对宿主细胞的体内安全性评估。(A, B) CQFC1在体内不改变宿主肝脏I期(A)和II期(B)解毒酶成分。每个样品扩增小鼠GAPDH以确保相同的cDNA输入。通过Image Lab软件对密度测量进行分析,并使用渐变色标度在热图中表示。热图中的每一个方框代表各自解毒酶对GAPDH表达归一化后的折叠变化的平均值;统计分析(均数±SEM和p值)见辅助信息表S6。使用GraphPad Prism软件(v8.0)生成重复实验的热图。(C, D) CQFC1不促进氧化应激,因为抗氧化剂[SOD和CAT (C;* p & lt;0.001 vs感染)]和脂质过氧化产物(D;* p & lt;0.001 vs感染)相对于未感染状态保持不变(C, D;p比;0.05 vs幼稚动物)。(E - g) CQFC1对小鼠器官也没有细胞毒性作用,从肝毒性特异性血清生物标志物酶的水平可以看出(E;* p & lt;0.001 vs感染),心脏毒性(F;* p & lt;0.001 vs感染)和肾毒性(G;* p & lt;0.001 vs感染)相对于未感染对照(E-G;p比;0.05 vs幼稚动物)。利用GraphPad Prism软件从每个实验组至少5只动物的累积数据中绘制散点图,一式两份(未感染:绿色;感染:红色;口服剂量2:天蓝色;肌肉注射剂量2(深蓝色)。采用单因素方差分析和Dunnett事后检验比较各组间均数的差异。这篇文章尚未被其他出版物引用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
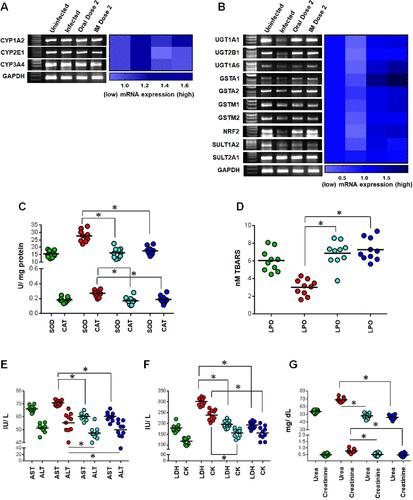
Correction to “Targeting the Trypanothione Reductase of Tissue-Residing Leishmania in Hosts’ Reticuloendothelial System: A Flexible Water-Soluble Ferrocenylquinoline-Based Preclinical Drug Candidate”
The authors have noticed that the GAPDH panel in Figure 2A and Figures 6A (Phase I detoxyfying enzymes) and 6B (Phase II detoxyfying enzymes) are identical, which has led us to rectify the GAPDH in the revised Figures 6A and 6B. The error in the figure occurred unintentionally during the electronic transfer of the original image files, which were placed in the final Figures 6A and 6B. The rectification of this error does not affect the results or conclusions, as the heat map created using a gradient color scale (densitometry) shown in the figure is indeed based on the original GAPDH (Figure 6A and 6B). All other data published in this article are correct. The supporting data included in the supplementary file (Table S6) will also remain the same as the calculations and the statistical analyses were performed using the original GAPDH. Figure 6. Assessment of the safety profile of CQFC1 toward the host cells, in vivo. (A, B) CQFC1 did not alter phase I (A) and phase II (B) detoxification enzyme components in the host liver, in vivo. Each sample was amplified for mouse GAPDH to ensure equal cDNA input. The densitometry was analyzed by Image Lab software and is represented in heat maps using a gradient color scale. Each square in the heat map represents the mean value of fold changes of respective detoxification enzymes normalized against the expression of GAPDH; the statistical analyses (mean ± SEM and p values) are presented in Supporting Information Table S6. GraphPad Prism software (v 8.0) was used to generate the heat maps from experiments performed in duplicate. (C, D) CQFC1 did not promote oxidative stress as the levels of the antioxidants [SOD and CAT (C; *p < 0.001 vs infection)] and lipid peroxidation products (D; *p < 0.001 vs infection) remained unchanged with respect to the uninfected state (C, D; p > 0.05 vs naive animals). (E–G) CQFC1 had no cytotoxic effect on murine organs also, as seen by the levels of serum biomarker enzymes specific for hepatotoxicity (E; *p < 0.001 vs infection), cardiotoxicity (F; *p < 0.001 vs infection), and nephrotoxicity (G; *p < 0.001 vs infection) with respect to uninfected controls (E–G; p > 0.05 vs naive animals). Scatter plots were prepared using GraphPad Prism software from the cumulative data obtained from at least 5 animals per experimental group in duplicate (uninfected: green; infected: red; oral dose 2: sky blue; and intramuscular dose 2: deep blue). One-way ANOVA followed by Dunnett’s post hoc test was used to compare the variations in means between experimental groups. This article has not yet been cited by other publications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


