脂质组学特征作为早发性肺癌的预测性生物标志物:一种风险预测模型的鉴定和发展
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
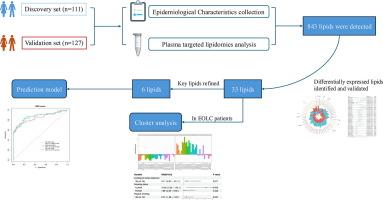
肺癌是全球癌症相关死亡的主要原因。虽然传统上与老年人有关,但早发性肺癌(EOLC)正在上升,特别是在亚洲,占全球病例的75.9%。现有的肺癌筛查指南主要侧重于老年人群,这可能导致年轻人错过早期发现的机会。鉴于其独特的临床特征,EOLC需要专门的研究和有针对性的干预措施。本研究旨在描述EOLC患者(18-49岁 岁)的脂质组学特征,并建立基于生物标志物的预测模型,以改善风险评估和早期发现。方法发现和验证组包括111例EOLC和127例非EOLC对照,年龄均为18-49岁 。结合logistic回归,对血浆样本进行靶向脂质组学分析,以确定差异表达的脂质种类。进行聚类和途径分析,以揭示和可视化鉴定的脂质的内部特征。采用LASSO-bootstrap回归法结合Boruta算法对关键脂质进行细化。随后采用随机森林模型建立了一个鲁棒的EOLC预测模型。结果共鉴定出843种脂质,检测到60种差异表达脂质,其中33种在验证集中得到验证。聚类分析显示,被动吸烟(OR: 3.11, 95% CI: 0.97-12.11)和当前吸烟(OR: 15.65, 95% CI: 2.55-142.10)与EOLC患者脂质代谢物谱升高相关。通过LASSO和Boruta方法对验证的脂质进行进一步的提炼,最终选择6种脂质纳入随机森林构建的预测模型。该模型在验证集中的曲线下面积(AUC)为0.874。结论:我们的研究确定了与EOLC风险相关的脂质组学特征,为肺癌预防策略提供了潜在的转化意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lipidomic signatures as predictive biomarkers for early-onset lung cancer: Identification and development of a risk prediction model
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide. While traditionally associated with older adults, early-onset lung cancer (EOLC) is rising, particularly in Asia, which accounts for 75.9% of global cases. Existing lung cancer screening guidelines primarily focus on older populations, which may result in missed opportunities for early detection in younger individuals. Given its distinct clinical characteristics, EOLC warrants dedicated research and targeted interventions.Objectives
This study aims to characterize the lipidomic profiles specific to EOLC patients (aged 18–49 years) and develop a biomarker-based predictive model to improve risk assessment and early detection.Methods
The discovery and validation sets included 111 EOLC cases and 127 non-EOLC controls, all aged 18–49 years. Targeted lipidomics analysis, combined with logistic regression, was performed on plasma samples to identify differentially expressed lipids species. Clustering and pathway analyses were conducted to uncover and visualize the internal signatures of the identified lipids. Key lipids were refined using the LASSO-bootstrap regression method combined with the Boruta algorithm. A random forest model was subsequently employed to develop a robust prediction model for EOLC.Results
A total of 843 lipids were identified, with 60 differentially expressed lipids detected, of which 33 were validated in the validation set. Cluster analysis revealed that passive smoking (OR: 3.11, 95% CI: 0.97–12.11) and current smoking (OR: 15.65, 95% CI: 2.55–142.10) were associated with elevated lipid metabolite profiles in EOLC patients. The validated lipids were further refined using LASSO and Boruta methods, which ultimately selected 6 lipids for inclusion in a prediction model constructed with random forest. This model achieved an area under the curve (AUC) of 0.874 in the validation set.Conclusion
Our study identified lipidomic signatures associated with the risk of EOLC, offering potential translational implications for lung cancer prevention strategies.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


