Cu(OTf)2催化含炔酚基双芳基硒化合成含硒螺环和菲
IF 2.5
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
介绍了一种在非氧化条件下由Cu(OTf)2促进、碱控制的炔与二硒化物选择性硒化反应合成硒化螺环己二烯酮和菲的简便易行的方法。该反应证明了良好的底物相容性,并提供了一系列中高产的芳基硒代螺环和菲分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
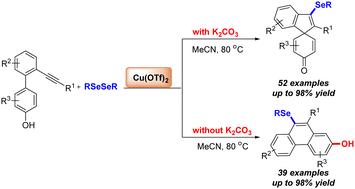
Divergent synthesis of selenide-containing spirocarbocycles and phenanthrenes through Cu(OTf)2-promoted selenylation of alkyne-containing phenol-based biaryls†
A convenient and practical approach for the synthesis of selenylated spirocyclohexadienones and phenanthrenes through the Cu(OTf)2-promoted, base-controlled selective selenylation of alkynes with diselenides under non-oxidative conditions is described. This reaction demonstrates excellent substrate compatibility and provides a series of aryl-selenide-substituted spirocarbocycles and phenanthrene molecules in moderate to high yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

New Journal of Chemistry
化学-化学综合
CiteScore
5.30
自引率
6.10%
发文量
1832
审稿时长
2 months
期刊介绍:
A journal for new directions in chemistry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


