二维层状钛酸盐纳米片(Ti2-x/4□x/4O4-x)的电催化效应□=空位,x = 0.67)修饰电极用于V3+/V2+氧化还原反应
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
二维(2D)电催化剂由于其高各向异性而产生的特殊电学性能,近年来引起了相当大的研究兴趣。二维材料的理想应用是催化必要的电化学反应以实现能量存储。为此,我们探索了剥离的二维层状钛酸盐纳米片(Ti2-x/4□x/4O4-x;□=空位,x = 0.67,本文简称LTNS)作为钒氧化还原液流电池(VRFB)的V3+/V2+氧化还原反应模型负极。采用浸提干燥法,在原始碳毡(P-CF)上涂上极少量(电极上负载量为~ 0.0043 wt%)的脱落LTNS胶体水悬浮液,覆盖碳纤维表面,从而制备LTNS@CF电极。与P-CF的光滑表面相比,LTNS@CF的形貌表现出被牢固附着的各向异性LTNS覆盖的相对粗糙的表面。通过电化学阻抗谱(EIS)、循环伏安法(CV)和VRFB单电池分析了这些电极对V3+/V2+氧化还原反应的电催化性能,与P-CF相比,LTNS@CF电极的电荷转移电阻(Rct)显著降低,V3+/V2+氧化还原反应动力学得到改善。这是由于LTNS具有理想的电催化性能:二维各向异性层状结构有利于电荷迁移并牢固附着在碳纤维表面,LTNS的负电荷为带正电的钒离子提供静电氧化还原反应位点,Ti-O键在强酸(H2SO4)电解质中的耐腐蚀性,其极性性质增强了电极的润湿性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
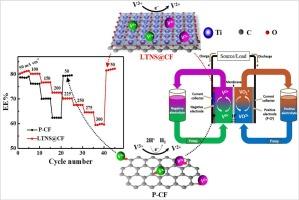
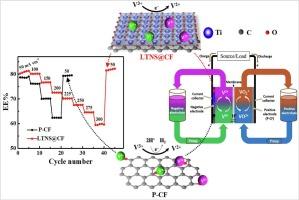
Electrocatalytic effects of two-dimensional (2D) layered titanate nanosheets (Ti2-x/4□x/4O4-x; □ = vacancy, x = 0.67) modified electrode for V3+/V2+ redox reactions
Two-dimensional (2D) electrocatalysts have attracted considerable recent research interest owing to the exceptional electrical properties arising from their high anisotropy. An ideal application of 2D materials is in the catalysis of essential electrochemical reactions for energy storage. To this effect, we explored the electrocatalytic properties of exfoliated 2D layered titanate nanosheets (Ti2-x/4□x/4O4-x; □ = vacancy, x = 0.67, herein abbreviated as LTNS) for V3+/V2+ redox reaction as a model negative electrode of vanadium redox flow battery (VRFB). The dip-withdraw-dry method was used to fabricate the LTNS@CF electrode by coating pristine carbon felt (P-CF) with a very small amount (∼ 0.0043 wt% loading amount on electrode) of exfoliated LTNS colloidal aqueous suspension equivalent to cover the surface of the carbon fibers. The morphology of LTNS@CF exhibited a relatively rough surface covered by the firmly attached anisotropic LTNS compared to the smooth surface of P-CF. The electrocatalytic properties of these electrodes for V3+/V2+ redox reactions were analyzed by electrochemical impendence spectroscopy (EIS), cyclic voltammetry (CV), and VRFB single-cell, with LTNS@CF showing significantly lower charge transfer resistance (Rct) and improved kinetics for V3+/V2+ redox reaction compared to that of P-CF. This is attributed to the concurrence of desired electrocatalytic properties of the LTNS: the 2D anisotropic layered shape that facilitates charge mobility and firm attachment to the surface of carbon fibers, the negative charge of LTNS that provides electrostatic redox reaction sites for the positively charged vanadium ions, corrosion resistance of Ti-O bond in strong acidic (H2SO4) electrolyte, and its polar nature that enhances the wettability of the electrode.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


