19f核磁共振化学位移编码肽筛选钾通道Kv1.3
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
19F核磁共振(19F- nmr)因其高灵敏度而成为蛋白质动力学研究和药物筛选的关键技术。在此,我们报告了一种基于19F-NMR化学位移的配体筛选策略,该策略使用含有不同含19f的非天然氨基酸的多肽配体靶向膜蛋白。五种不同的19F标记的非天然氨基酸(3FF、4FF、5FW、6FW和7FW)具有不同的19F化学位移值,用于化学合成多种毒素肽,这些毒素肽可能结合并抑制自身免疫性疾病相关钾通道Kv1.3的传导功能。从kv1.3肽复合物中竞争洗脱的肽的19F NMR弛豫滤波一维光谱直接揭示了肽的高亲和力结合,并通过膜片钳电生理分析验证了这一点。这种基于19F-NMR化学位移的编码肽筛选方法可以直接扩展到针对膜蛋白的大规模肽库筛选。本文章由计算机程序翻译,如有差异,请以英文原文为准。
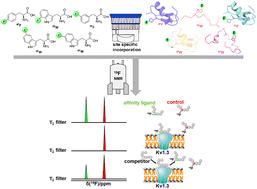
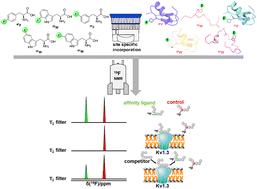
19F NMR chemical shift encoded peptide screening targeting the potassium channel Kv1.3†
19F nuclear magnetic resonance (19F-NMR) is a pivotal technique for protein dynamic studies and drug screening because of its high sensitivity. Herein, we report a 19F-NMR chemical shift-based ligand screening strategy targeting membrane proteins using multiple peptide ligands containing different 19F-incorporating unnatural amino acids. Five different 19F-labelled unnatural amino acids (3FF, 4FF, 5FW, 6FW and 7FW) with distinctive 19F chemical shift values were applied to chemically synthesize multiple toxin peptides, which can potentially bind to and inhibit the conductance function of the autoimmune disease-related potassium channel Kv1.3. The 19F NMR relaxation-filtered one-dimensional spectra of competitively eluted peptides from the Kv1.3-peptide complex directly revealed the high-affinity binding of the peptides, which was verified using patch-clamp electrophysiological analysis. This 19F-NMR chemical shift-based encoded peptide screening method can be directly extended for large-scale peptide library screening targeting membrane proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


