钴/铈基丙烷脱氢催化剂中邻近依赖的氧化物-负载相互作用
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
coox基丙烷脱氢催化剂引起了广泛的研究兴趣。然而,钴价态的不确定性对其性能的提高提出了挑战。本文描述了邻近依赖的Co2+在钴/铈基非氧化丙烷脱氢催化剂中的关键作用。通过结合透射电镜和原位光谱,我们发现由CoOx-CeOx合作界面稳定的Co2+,而不是金属Co0,负责激活丙烷的C-H键。在CoOx和CeOx紧密接触的1Co/20CeAl催化剂中,Co2+含量最高,丙烯的时空收率最高,选择性高达86%。动力学研究表明,邻近依赖的氧化物-载体相互作用调节了Co0/Co2+比率,导致速率决定步骤从1Co/Al中的第一个C-H键激活转变为1Co/20CeAl中的第二个C-H键激活。本研究强调利用氧化物-载体相互作用来优化催化性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
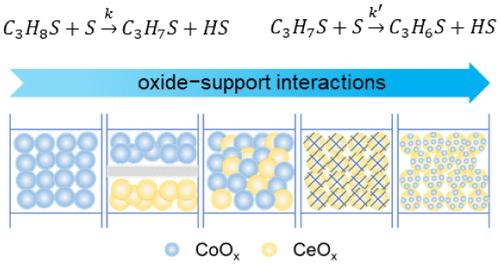
Proximity-Dependent Oxide–Support Interactions in Cobalt/Ceria-Based Catalysts for Propane Dehydrogenation
The CoOx-based catalyst has attracted extensive research interest for propane dehydrogenation. However, enhancing its performance has been challenged by uncertainties surrounding the valence states of cobalt. This paper describes the pivotal role of proximity-dependent Co2+ in cobalt/ceria-based catalysts for nonoxidative propane dehydrogenation. By combining transmission electron microscopy and in situ spectroscopies, we discovered that Co2+, stabilized by the cooperative CoOx–CeOx interface, rather than metallic Co0, is responsible for activating the C–H bonds of propane. For the 1Co/20CeAl catalyst, where CoOx and CeOx are in intimate contact at the nanoscale, the highest Co2+ content was achieved, leading to the highest space–time yield (STY) of propylene with a high selectivity of 86%. Kinetic studies indicate that the proximity-dependent oxide–support interaction mediates the Co0/Co2+ ratios, resulting in a shift in the rate-determining step from the first C–H bond activation in 1Co/Al to the second C–H bond activation in 1Co/20CeAl. This study emphasizes the utilization of oxide-support interactions to optimize catalytic performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


