探索固相反应机理,阐明通过热化学耦合活化煤矸石中的硅质矿物形成有效二氧化硅的机理
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
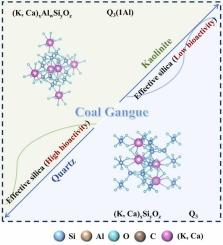
煤矸石(CG)提供了可转化为有效二氧化硅的硅质矿物的丰富来源。本研究旨在深入了解石英(Qtz)和高岭石(Kln)在热化学耦合活化转化为有效二氧化硅过程中的演化机理。通过两种硅质矿物的结构转化和热力学计算,建立了有效二氧化硅形成的相图模型,为固相反应的热力学研究提供了科学依据。研究表明,在热化学耦合活化过程中,K2CO3 的活化效率优于 CaCO3,Kln 的活化效率高于 Qtz。值得注意的是,当煅烧温度达到 850 ℃ 时,添加了 K2CO3 的 Kln 系统的活化效率可超过 50%。有效二氧化硅性质和结构的表征与分析表明,具有(K,Ca)xSiyOz 的矿物中存在低生物活性有效二氧化硅,对应于 Q3 结构;而具有(K,Ca)xAlwSiyOz 的矿物中存在高生物活性有效二氧化硅,对应于 Q3(1Al) 结构。最后,我们利用 FactSage 热力学计算软件进一步预测了固相反应的相变过程。模拟结果与实验数据高度吻合,为二氧化硅的有效合成和工艺优化提供了有力支持。该研究为理解硅质矿物在CG内转化为有效二氧化硅的多途径、多相反应机理提供了理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring solid-phase reaction mechanism to elucidate the formation of effective silica through thermal–chemical coupled activation of siliceous minerals in coal gangue
Coal gangue (CG) provides an abundant source of siliceous minerals that can be converted into effective silica. This study aimed to gain a deeper understanding of the evolutionary mechanism of quartz (Qtz) and kaolinite (Kln), in the conversion process of thermal–chemical coupled activation to effective silica. The structure transformation of two types of siliceous minerals and thermodynamic calculation were used to develop a phase diagram model for the formation of effective silica, and then provide a scientific basis for the thermodynamic study of solid-phase reactions. We showed that K2CO3 outperformed CaCO3 in activation efficiency in the thermal–chemical coupled activation process, and Kln had a higher activation efficiency than Qtz. Notably, when the calcination temperature reaches 850 °C, the activation efficiency of the Kln system with added K2CO3 could exceed 50%. Characterization and analysis of the effective silica properties and structure revealed that low bioactivity effective silica existed in the minerals with (K, Ca)xSiyOz, corresponding to the Q3 structure; while high bioactivity effective silica existed in the minerals with (K, Ca)xAlwSiyOz, corresponding to the Q3(1Al) structure. Finally, we used FactSage thermodynamic calculation software to further predict the phase transformation process of the solid-phase reaction. The simulation results highly matched the experimental data, providing strong support for effective silica synthesis and process optimization. This study provides theoretical basis for understanding the multi-pathway, multi-phase reaction mechanisms of siliceous minerals transforming into effective silica within CG.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


