无水盐用于无腐蚀性铝电池电解质
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
商用三氟酸铝和双(三氟甲烷磺酰)亚胺铝盐是水合物,它使水中含有电解质,通过Al(OH)3的形成使电极钝化。我们通过酸碱反应生产无水盐,由此产生的无水电解质表现出增强的电化学活性,为铝电池的发展铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
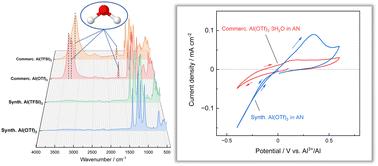
Anhydrous salts for non-corrosive aluminium battery electrolytes†
Commercial aluminium triflate and aluminium bis(trifluoromethanesulfonyl)imide salts are hydrates, which renders water containing electrolytes that passivate electrodes through Al(OH)3 formation. By acid–base reactions we produce anhydrous salts and the resulting anhydrous electrolytes demonstrate enhanced electrochemical activity, paving the way for aluminium battery development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


