喹啉的逐步脱芳化/硝化/对映选择性同烯酸酯反应生成c3 -硝基取代四氢喹啉。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们描述了喹啉的逐步1,2-还原脱芳/选择性c3 -硝化和随后的催化对映选择性齐烯酸酯加成反应,使用NHC催化剂策略构建n -乙酰3,4-二取代四氢喹啉,收率高,具有非常高的非映和对映选择性(dr bbb99: 1, ee高达>99%)。本文还实现了一种以易得的喹啉为原料合成3-硝基喹啉的高效无金属无碱方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
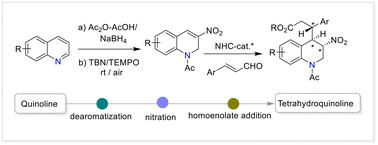
A stepwise dearomatization/nitration/enantioselective homoenolate reaction of quinolines to construct C3-nitro-substituted tetrahydroquinolines†
Herein, we describe a stepwise 1,2-reductive dearomatization/selective C3-nitration of quinoline and a subsequent catalytic enantioselective homoenolate addition reaction using a NHC catalyst strategy to construct N-acetyl 3,4-disubstituted tetrahydroquinoline in good yields with remarkably high diastereo- and enantioselectivities (dr >99 : 1, ee up to >99%). An efficient metal- and base-free method for 3-nitroquinoline synthesis from readily accessible quinoline has also been realized.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


