氧空位介导的电子加速和催化氧化协同增强铁锰二元吸附剂对砷的去除
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
缺陷工程金属氧化物具有通过改进As2O3吸附和氧化过程来增强砷捕获的巨大潜力。然而,这种增强的机制,特别是氧空位(OV)和内在氧化方案所激发的电子效应,目前尚不清楚。实验和理论研究表明,柠檬酸刻蚀Fe-Mn二元吸附剂产生的OV显著促进了铁和锰在氧化环境下对As(III)的氧化。电子顺磁共振和吸附特性表明,OV发生了选择性的砷吸附,使其受限吸附活性提高了58.9%。电化学和静电电位研究表明,OV在表面产生了独特的高活性电位场,其特点是提高了电导率和优越的氧化还原性能。界面电子调节使OV在离子键和共价键增强以及电子传递通道桥化的驱动下,实现了砷吸附的定向电子重分配。OV的催化作用优先促进了物理吸附向化学吸附的转化,从而增强了砷的动态吸附。最后阐明了OV对砷的内在强化氧化途径。OV遵循氧温程序解吸(O2-TPD)和Mars-Maessen机制,促进了表面晶格氧的迁移,重建了块状晶格氧的输运通道,有效降低了决定速率的阶能垒(超过2.066 eV),促进了As3+氧化为As5+。这些发现表明,氧空位工程可以在宏观和原子水平上有效地开发和利用高效的气态砷吸附剂。环境影响砷(Ⅲ)因其极高的毒性、挥发性、生物蓄积性和致癌性而受到全球关注。气相砷最终会污染土壤或水,严重危害人类健康和生态系统稳定。近年来,由于资源利用不到位,环保意识不强,导致特定地区砷污染严重,发生多起砷中毒事件。本研究为通过吸附剂表面氧空位工程技术推进高效烟气除砷技术提供了新的视角。本文首次报道了Fe-Mn二元吸附剂通过氧空位介导的电子加速和催化氧化协同增强除砷效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
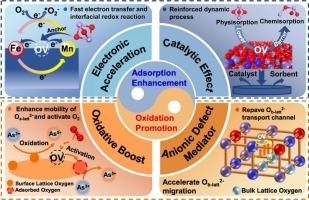
Synergistic enhancement of arsenic removal by Fe-Mn binary sorbent through oxygen vacancy-mediated electron acceleration and catalytic oxidation
Defect-engineered metal oxides hold great potential for enhancing arsenic capture through improved As2O3 adsorption and oxidation processes. However, the mechanisms underlying this enhancement, particularly the electronic effects stimulated by oxygen vacancy (OV) and the intrinsic oxidation schemes, are poorly understood. In this work, experimental and theoretical studies reveal that OV generated through citric acid etching of Fe-Mn binary sorbents markedly boosts the oxidation of As(III) by iron and manganese under oxic environments. Electron paramagnetic resonance and adsorption characteristics demonstrate that selective arsenic adsorption occurs at OV, leading to a 58.9 % enhancement in confined adsorption activity. Electrochemical and electrostatic potential investigations demonstrate that OV generates a distinctive high-activity potential field on the surface, characterized by improved electrical conductivity and superior redox properties. Interfacial electron regulation enables OV to achieve directional electron redistribution for arsenic adsorption, driven by strengthened ionic and covalent bonds as well as bridged electron transport channels. The catalytic effect imparted by OV preferentially facilitates the conversion from physisorption to chemisorption, resulting in reinforced dynamic arsenic adsorption. Ultimately, the intrinsically intensified oxidative pathways for arsenic by OV were elucidated. Following the oxygen temperature programming desorption (O2-TPD) and Mars-Maessen mechanism, OV facilitates the migration of surface lattice oxygen and reestablishes transport channels for bulk lattice oxygen, effectively lowering the rate-determining step energy barrier (exceeding 2.066 eV) and promoting the oxidation of As3 + to As5+. These findings demonstrate that oxygen vacancy engineering can be effectively implemented in developing and utilizing efficient gaseous arsenic sorbents at both macroscopic and atomic levels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


