铁氧化还原蛋白激发的立体异构体fe2cn -桥- s双原子催化剂增强酸性氧还原反应
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
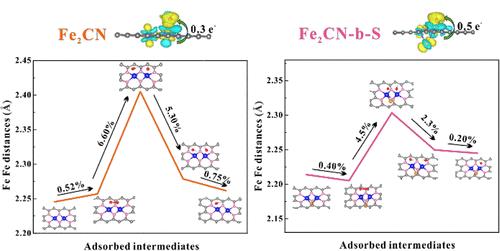
双金属中心催化剂(DMC)以其独特的结构为加速氧还原反应(ORR)注入了新的活力。金属活性中心协调环境组成和空间结构的调控也为优化性能提供了契机。本文基于铁氧还蛋白的仿生Fe-S簇结构,成功构建了立体异构体fe2cn -桥- s(简称Fe2CN-b-S)催化剂。相邻的铁双原子巧妙地削弱了O-O键,形成了过氧化物桥状的吸附结构。S原子的加入精心构建了活性Fe位的立体构型,从而诱导了更大的结构变形张力和铁d带中心的向下移动。这些因素共同促进OH*中间体的释放。同时,S的合理空间构型可以促进Fe双原子活性中心的最优刚性结构,提高ORR反应过程的稳定性。因此,半波电位为0.865 V的Fe2CN-b- s催化剂表现出比Fe2CN更强的ORR活性。本研究为元素组成和几何排列对氧还原催化活性增强的共同调控提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ferredoxin-Inspired Stereoisomeric Fe2CN-Bridge-S Dual Atom Catalyst for Enhanced Acid Oxygen Reduction Reaction
The bimetallic center catalyst (DMC) injects new vitality into the accelerated oxygen reduction reaction (ORR) due to its unique structure. The regulation of the coordination environment composition and spatial structure of metal active centers also provides opportunities for optimizing the performance. Herein, we have successfully constructed stereoisomeric Fe2CN-bridge-S (abbreviated as Fe2CN-b-S) catalysts based on the biomimetic Fe–S cluster structure of ferredoxin. Adjacent Fe dual atoms skillfully weaken the O–O bond, crafting a peroxide bridge-like adsorption configuration. The incorporation of S atoms meticulously constructs the stereo configuration of active Fe sites, thereby inducing greater structural deformation tension and a downward shift in the Fe d-band center. These factors collectively facilitate the release of the OH* intermediate. Meanwhile, the reasonable spatial configuration of S can promote the optimal rigid structure of Fe diatomic active centers, improving the stability of the ORR reaction process. Thus, the Fe2CN-b-S catalyst, which has a half-wave potential of 0.865 V, demonstrates superior ORR activity in comparison to Fe2CN. This study offers a perspective on the joint regulation of elemental composition and geometric arrangement for enhanced catalytic activity in oxygen reduction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


