Fe3O4@Bi2S3纳米颗粒介导的mri引导下通过外磁对原位胶质母细胞瘤的精确放射增敏
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
肿瘤的放射耐药和放疗过程中正常组织的过度损伤限制了胶质母细胞瘤放射治疗的临床应用,影像引导下的放射增敏有望实现精准放射治疗。然而,由于血脑屏障的限制和肿瘤靶向性差,放射增敏剂在胶质母细胞瘤中的治疗效果并不理想。在此,Fe3O4@Bi2S3纳米颗粒包被胶质母细胞瘤细胞膜(简称FBM)被设计用于致敏rt。FBM通过外部磁性和同源粘附能力在肿瘤内精确积累。之后,FBM在电离辐射和肿瘤微酸性环境中释放高z原子(铋),这些环境与电离辐射相互作用产生高密度的二次辐射,导致辐射剂量沉积增强。同时,FBM产生活性氧,积累脂质过氧化和Fe2+,消耗谷胱甘肽,下调谷胱甘肽过氧化物酶4,激活铁下沉。值得注意的是,fbm介导的外磁RT在原位胶质母细胞瘤模型中的肿瘤生长抑制率提高到75.49%。此外,具有磁共振成像性能的FBM在肿瘤诊断和治疗监测中显示出潜在的应用前景,从而减少对邻近正常组织的损伤,实现mri引导下的精确rt。因此,新型多功能纳米平台为激活铁上沉降诱导的图像引导放射增敏提供了可能,从而为胶质母细胞瘤的放射治疗提供了一种有效的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
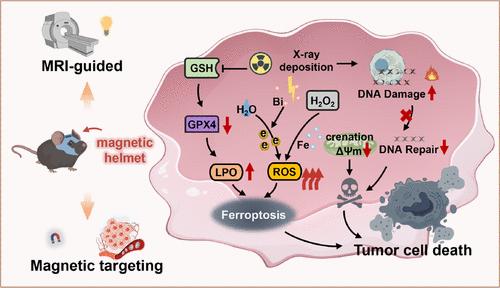
Fe3O4@Bi2S3 Nanoparticles Mediated MRI-Guided Precision Radiosensitization for Orthotopic Glioblastoma via External Magnetism
Radioresistance in tumors and the excess damage in normal tissues during radiotherapy (RT) restrict the clinical application of glioblastoma RT. Image-guided radiosensitization is hopefully adopted to achieve precision RT. Nevertheless, the therapeutic effect of radiosensitizers in glioblastoma is unsatisfactory due to limitations of the blood–brain barrier and poor tumor targeting. Herein, Fe3O4@Bi2S3 nanoparticles coated with a glioblastoma cell membrane (denoted as FBM) have been designed to sensitize RT. FBM accumulates precisely within the tumors via external magnetism and homologous adhesion capability. Afterward, FBM releases high-Z atoms (Bismuth) in ionizing radiation and tumor micro acidic environments that interact with ionizing radiation to generate high densities of secondary radiation, which leads to enhanced radiation dose deposits. Simultaneously, FBM generates reactive oxygen species, accumulates lipid peroxidation and Fe2+, depletes glutathione, and downregulates glutathione peroxidase 4 to activate ferroptosis. Notably, the tumor growth inhibition rate of FBM-mediated RT via external magnetism increases to 75.49% in the orthotopic glioblastoma model. Besides, FBM with magnetic resonance imaging performance shows the potential application in tumor diagnosis and therapy surveillance, thereby reducing damage to adjacent normal tissues and realizing MRI-guided precision RT. Hence, the novel multifunctional nanoplatform offers the potential for image-guided radiosensitization induced by activating ferroptosis, thus presenting an efficient radiotherapeutic approach for glioblastoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


