靶向降解EGFR突变通过自身递送纳米protacs促进肿瘤协同免疫治疗
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
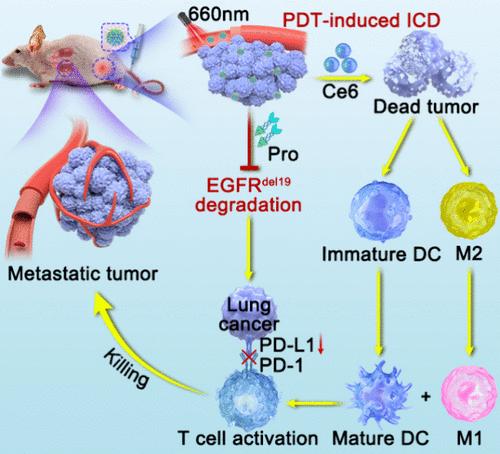
蛋白水解靶向嵌合体(Proteolysis targeting chimera, PROTAC)最近成为一种有前途的选择性降解靶蛋白的策略,用于治疗各种疾病。但其疏水性强、膜透性差、体内分布非特异性,生物利用度低,极大地限制了其应用。在这项研究中,利用基于吉非替尼的PROTAC和光敏剂,开发了自我递送的PROTAC纳米颗粒(称为CP NPs),以有效降解突变的表皮生长因子受体(EGFR),这是细胞生长和存活的关键激酶,同时引发光动力治疗和免疫治疗。制备的NPs增强了PROTACs在肿瘤中的积累,导致EGFR突变的选择性降解和程序性细胞死亡蛋白配体1水平的降低,从而减轻肿瘤的免疫抑制和免疫耐受。此外,在激光照射下,负载的光敏剂引发了强效的光动力治疗效果并诱导免疫原性细胞死亡,这与PROTACs协同作用,引发了强大的抗肿瘤免疫应答。在小鼠肺癌模型中,原发性、远处和肺转移性肿瘤被显著抑制。这项工作强调了纳米PROTAC在降解靶蛋白和促进联合光动力免疫治疗方面的潜力,从而扩大了PROTAC在癌症治疗中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeted Degradation of EGFR Mutations via Self-Delivery Nano-PROTACs for Boosting Tumor Synergistic Immunotherapy
Proteolysis targeting chimera (PROTAC) has recently emerged as a promising strategy to selectively degrade target proteins in the treatment of various diseases. However, it has low bioavailability due to strong hydrophobicity, poor membrane permeability, and nonspecific distribution in vivo, which greatly limits its application. In this study, self-delivery PROTAC nanoparticles (designated as CP NPs) integrating gefitinib-based PROTACs and photosensitizers were developed to efficiently degrade mutated epidermal growth factor receptor (EGFR), a crucial kinase for cell growth and survival, while simultaneously triggering photodynamic therapy and immunotherapy. The prepared NPs enhanced the tumor accumulation of PROTACs, which led to the selective degradation of EGFR mutations and a reduction in programmed cell death protein ligand 1 levels, thereby alleviating tumor immunosuppression and immune tolerance. Moreover, under laser irradiation, the coloaded photosensitizers triggered potent photodynamic therapy effects and induced immunogenic cell death, which worked synergistically with PROTACs toward eliciting a robust antitumor immune response. In a mouse model of lung cancer, primary, distant, and lung metastatic tumors were significantly suppressed. This work highlights the potential of nano-PROTACs for degrading target proteins and facilitating combination photodynamic immunotherapy toward expanding PROTAC applications in cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


