基于姜黄素的BF3荧光检测响应表面,快速可逆的方法
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
一种新型姜黄素(CCMoid)的战略性设计,称为PA,含有芘单元和末端羧基,为其在表面上的有效固定和作为光学化学传感器的潜在用途提供了必要的工具。为此,我们的工作为制备具有荧光响应和可重用性的基于ccmoid的活性表面提供了一种强大的方法。CCMoid的共价固定是通过PA的酸性基团与先前功能化底物的咪唑端反应获得的。通过这种方法,利用微接触印刷获得了PA的荧光图案表面,由于CCMoid的芘基团,可以在可见光区观察到其发射。此外,还分析了BF3分子(在溶液和气相中)与固定在表面的PAs的酮烯醇部分的配位。BF3能够改变基于ccmoids的表面的光学特性,导致近红外辐射,这是一个快速和可逆的过程。这种能力是最终协调系统所固有的,而不是其他硼基分子,提供独特的响应和传感表面。本文章由计算机程序翻译,如有差异,请以英文原文为准。
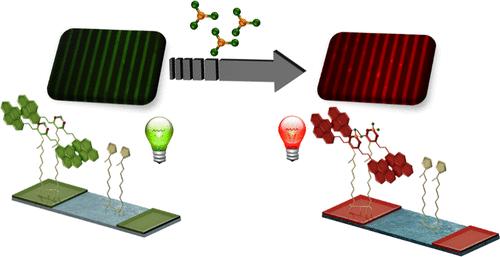
Curcuminoid-Based Responsive Surfaces for Fluorescent BF3 Detection, a Fast and Reversible Approach
The strategic design of a novel curcuminoid (CCMoid), termed PA, containing pyrene units and a terminal carboxylic group provides the necessary tools for its efficient immobilization on surfaces and its potential use as an optical chemosensor. To this end, our work provides a robust methodology for the preparation of CCMoid-based active surfaces with a fluorescent response and reusability. The covalent immobilization of the CCMoid is obtained by the reaction of the acidic groups of PA and the imidazole ends of the previously functionalized substrates. In this way, fluorescent patterned surfaces of PA, whose emission could be observed in the visible region thanks to the pyrene groups of the CCMoid, were obtained using microcontact printing. In addition, the coordination of BF3 molecules (in solution and in gas phase) with the keto–enol moiety of the PAs anchored on the surfaces has been analyzed. The ability of BF3 to modify the optical properties of the CCMoids-based surfaces, leading to emissions in the near-IR, has been identified as a fast and reversible process. Such ability is intrinsic to the final coordinated system and not to other boron-based molecules, providing unique response and sensing surfaces.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


