妊娠期是环境空气污染暴露对产妇产后代谢组的易感期
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
妊娠期是空气污染暴露对母体长期代谢影响的潜在关键窗口期。然而,人们对空气污染与孕产妇代谢健康之间的潜在早期代谢机制知之甚少。我们纳入了544名怀孕的墨西哥妇女,她们在怀孕期间的环境PM2.5水平和非靶向血清代谢组学研究了怀孕期间PM2.5暴露(总体和每月)与产后代谢物之间的关系,实施了fdr调整的稳健线性回归,控制了协变量。途径富集分析(Reactome和MetaboAnalyst)以及胎儿性别和叶酸补充对效果的改变也进行了评估。妊娠期间较高的PM2.5暴露水平与胆汁酸和氨基酸升高、甘油磷脂失调或脂肪酰基水平降低有关(FDR <;0.05),以及其他代谢产物。在妊娠早期至中期观察到月度PM2.5对代谢物易感性的潜在关键窗口(FDR <;0.005)。主要研究结果与胎儿性别和叶酸补充水平一致。与pm2.5阳性代谢物相关的代谢途径表明胆汁酸、膳食脂质和跨膜转运代谢富集,而对于pm2.5阴性代谢物相关的代谢途径,我们发现涉及脂肪生成、肠促胰岛素肽激素、GLP-1、ppar - α和脂肪酸受体(FDR <;0.05)。PM2.5暴露在怀孕期间,特别是在妊娠早期,改变了产妇产后脂质和氨基酸代谢。本文章由计算机程序翻译,如有差异,请以英文原文为准。
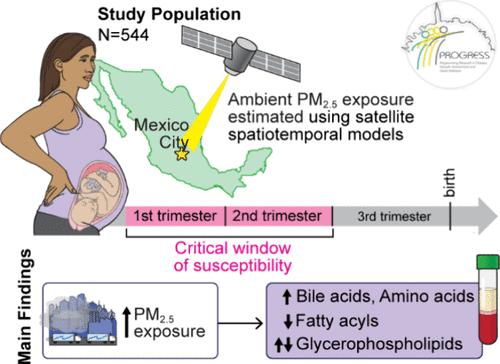
Pregnancy as a Susceptible Period to Ambient Air Pollution Exposure on the Maternal Postpartum Metabolome
Pregnancy is a potential critical window to air pollution exposure for long-term maternal metabolic effects. However, little is known about potential early metabolic mechanisms linking air pollution to maternal metabolic health. We included 544 pregnant Mexican women with both ambient PM2.5 levels during pregnancy and untargeted serum metabolomics to examine associations between pregnancy PM2.5 exposure (overall and monthly) and postpartum metabolites, implementing FDR-adjusted robust linear regression controlling for covariates. Pathway enrichment analyses (in Reactome and MetaboAnalyst) and effect modification by fetal sex and folic acid supplementation were also evaluated. Higher PM2.5 exposure levels throughout pregnancy were associated with higher bile acids and amino acids, dysregulated glycerophospholipids, or lower fatty acyl levels (FDR < 0.05), among other metabolites. Potential critical windows of susceptibility to monthly PM2.5 on metabolites were observed in early to midpregnancy (FDR < 0.005). Main findings were consistent by strata of fetal sex and folic acid supplementation. Metabolic pathways corresponding to positive PM2.5-metabolite associations indicated enriched bile acid, dietary lipid, and transmembrane transport metabolism, whereas for negative PM2.5-metabolite associations, we identified altered pathways involving adipogenesis, incretin peptide hormone, GLP-1, PPAR-alpha, and fatty acid receptors (FDR < 0.05). PM2.5 exposures during pregnancy, especially in early gestation, altered maternal postpartum lipids as well as amino acid metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


