一种新型β-磺胺基乙烯基磺酰氟化合物立体选择性结构的简单方案
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在有机碱的存在下,在2-溴丙基-2-烯-1-磺酰氟(BPESF)上加成巯基已成功地用于合成高度功能化的磺胺基乙烯基磺酰氟。该反应具有底物范围广、反应条件温和、原子经济性高、分离收率可达100%、立体选择性强等特点,在化学生物学和药物化学等领域具有重要的应用价值。进一步的产品多样化导致了胺基磺酰氟的构建。本文章由计算机程序翻译,如有差异,请以英文原文为准。
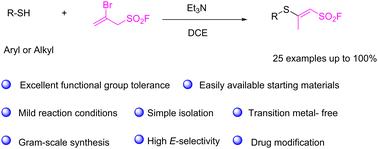
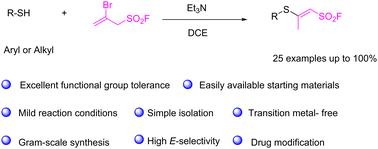
A simple protocol for stereoselective construction of novel β-sulfanyl vinyl sulfonyl fluorides†
The addition of thiols to 2-bromoprop-2-ene-1-sulfonyl fluoride (BPESF) in the presence of an organic base has been successfully employed for the synthesis of highly functionalized sulfanyl vinyl sulfonyl fluorides. This reaction features a broad substrate scope, mild reaction conditions, high atom economy, good to excellent isolated yields of up to 100%, and remarkable stereoselectivity, making it valuable for applications in chemical biology and medicinal chemistry. Further product diversification resulted in the construction of enaminyl sulfonyl fluorides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


