邻乙烯基异氰酸酯和芳基重氮四氟硼酸酯的光氧化还原自由基环化合成2,4-二取代喹啉
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
喹啉类化合物广泛存在于功能化合物中,是含氮杂环化合物的重要组成部分。Herin是邻乙烯基异氰酸酯和芳基重氮四氟硼酸酯的光化学自由基环化反应,已被报道生成2,4-二芳基喹啉。在室温下,易得的芳基重氮盐被用作芳基自由基前体。这种方法具有良好的官能团耐受性和底物适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
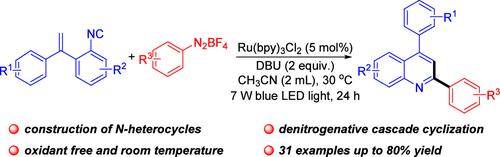
Photoredox Radical Cyclization of o-Vinylaryl Isocyanides and Aryldiazonium Tetrafluoroborates for the Synthesis of 2,4-Disubstituted Quinolines
Quinolines, a significant part of nitrogen-containing heterocycles, are widely found in functional compounds. Herin, a photochemical radical cyclization reaction of o-vinylaryl isocyanides and aryldiazonium tetrafluoroborates, has been reported to build 2,4-diaryl quinolines. Readily accessible aryl diazonium salts were utilized as aryl radical precursors at room temperature. This approach allowed good functional group tolerance and substrate applicability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


