可持续地制造用于制药的高附加值萘醌†。
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
目前全球正在进行的最关键和最重要的研究是以更环保的方式将丰富的原料转化为具有材料和药用价值的支架。这就需要适当选择原料,并制定系统的绿色合成战略,将其转化为有价值的实体。最具吸引力和最丰富的原料之一是法律。然而,从lawsones的动态互变异构体中选择性去除烯醛羟基以获得1,2-和/或1,4-萘醌仍然是一个挑战,它们具有广泛的合成应用。在此,我们报道了无金属,化学/区域选择性高产氢脱羟基(HDH)和氢脱氯(HDC)策略,其中丰富的3-烷基lawsones在微波照射下用AcOH水溶液HI处理几分钟,以获得大量具有重要物质和药用价值的1,2-和1,4-萘醌。我们还报道了利用目前开发的HDH作为关键反应,短时间合成具有重要医学意义的药物分子,如脱氧新cryptotanshinone, miltirone和维生素k3 (menadione)及其类似物。原料lawsone和3-烷基lawsone的商业化/易得性,水合酸和乙酸的生物友好性,烯醇羟基羟基的完全化学/区域选择性,底物范围广,产量多,合成应用广泛,药物分子合成时间短,这些都是与之前的1,2-萘醌合成相比,这项工作的主要吸引力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
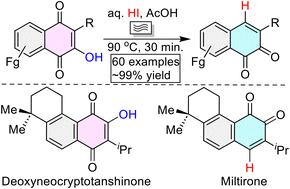
Sustainable construction of value-added naphthoquinones for pharmaceuticals†
The most crucial and essential ongoing global research is to convert the abundantly available feedstock into materially and medicinally valuable scaffolds in a greener mode. This requires the proper selection of feedstock and the development of systematic green synthetic strategies to convert it into valuable entities. One of the most attractive and abundant feedstocks is lawsones. However, the selective removal of the enolic hydroxy group from the dynamic tautomers of lawsones to access 1,2- and/or 1,4-naphthoquinones, which have plenty of synthetic applications, remains a challenge. Herein, we report metal-free, chemo-/regio-selective high-yielding hydrodehydroxylation (HDH) and hydrodechlorination (HDC) strategies in which the abundantly available 3-alkyllawsones were treated with aqueous HI in AcOH under microwave irradiation for a few minutes to access a vast library of materially and medicinally important 1,2- and 1,4-naphthoquinones. We also reported the short total synthesis of medicinally important drug molecules, such as deoxyneocryptotanshinone, miltirone, and vitamin-K3 (menadione) and their analogues, using the currently developed HDH as a key reaction. The commercial/easy availability of the feedstock lawsone and 3-alkyllawsones, bio-friendly nature of aqueous hydriodic acid and acetic acid, complete chemo-/regio-selectivity of hydrodehydroxylation of the enolic-hydroxy groups, huge substrate scope, quantitative yields, large synthetic applications, and the short synthesis of medicinal molecules are the key attractions of this work compared to the previous synthesis of 1,2-naphthoquinones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


