探讨碳酸二甲酯氧化Pt/CeO2和Pt/Al2O3的反应途径:来自原位漂移和DFT计算的证据
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
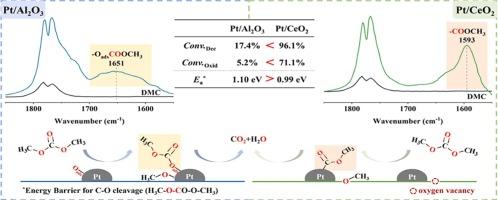
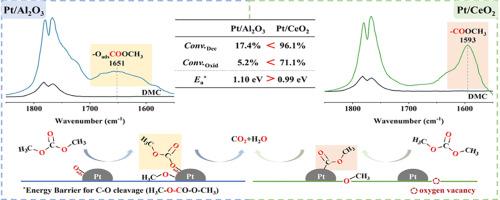
尽管与其他挥发性有机化合物(VOCs)相比,碳酸二甲酯(DMC)表现出较低的光化学活性,但由于其在固定和移动应用中的需求显著增加,它仍然需要消除。催化氧化已成为控制DMC排放的重要技术,但其反应机理尚不清楚。本研究以Pt/CeO2和Pt/Al2O3为模型催化剂,研究了DMC的氧化机理。原位漂移和DFT计算表明,DMC在Pt/Al2O3和Pt/CeO2催化剂上的催化氧化都始于羧基内C-O键的断裂,随后发生氧化。值得注意的是,Pt/CeO2表面氧空位的存在有利于DMC的分解,导致Pt/CeO2上C-O键劈裂的能垒比Pt/Al2O3上低,这是其具有优异催化活性的原因。本研究不仅对DMC的催化氧化过程有了初步的了解,而且对不同活性氧对催化反应的影响也有了初步的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Probing the reaction pathway of dimethyl carbonate oxidation on Pt/CeO2 and Pt/Al2O3: Evidence from in situ DRIFTS and DFT computation
Although dimethyl carbonate (DMC) exhibits low photochemical activity compared with other volatile organic compounds (VOCs), it still necessitates elimination due to its significant increasing demand in both stationary and mobile applications. Catalytic oxidation has emerged as an important technique for controlling DMC emissions, yet the reaction mechanism of this process remains unclear. In this study, we utilized Pt/CeO2 and Pt/Al2O3 as model catalysts to investigate the oxidation mechanism of DMC. In situ DRIFTS and DFT calculations reveal that the catalytic oxidation of DMC on Pt/Al2O3 and Pt/CeO2 catalysts both initiate with the cleavage of the C-O bond within the carboxyl group, followed by subsequent oxidation. Notably, the presence of oxygen vacancies on the Pt/CeO2 surface facilitates the decomposition of DMC, resulting in a lower energy barrier for C-O bond cleavage on Pt/CeO2 compared to that on Pt/Al2O3, which accounts for its superior catalytic activity. The present work not only provides an understanding of the catalytic oxidation process of DMC but also offers preliminary insights into the influence of different active oxygen species on the catalytic reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


