氨基功能化MIL-101(Fe)-NH2作为选择性污染物降解的高效过氧乙酸活化剂:揭示给电子配体在Fe(IV)生成中的作用
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
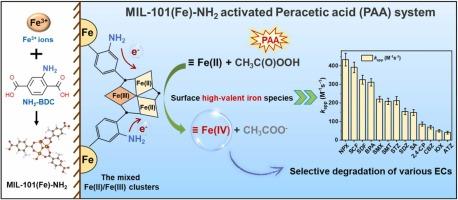
基于过氧乙酸的高级氧化工艺(PAA-AOPs)产生各种活性自由基,在降解新兴污染物(ECs)方面受到了广泛关注。然而,非选择性自由基基PAA-AOPs经常受到水基质组分的干扰,导致其去除污染物的效率较低。本研究探索了氨基(NH2)功能化金属有机框架(MIL-101(Fe)-NH2)作为PAA活化的多相催化剂,使高价铁(Fe)-氧(Fe(IV))能够在30分钟内有效降解ECs(80 - 100%)。MIL-101(Fe)-NH2中的Fe(II)团簇在Fe(IV)生成过程中起关键作用,并受供电子-NH2基团的调控。清道夫和探针实验证实Fe(IV)是ec降解的主要反应物质。密度泛函数理论计算表明,生成Fe(IV)的四电子转移比生成有机自由基(如CH3COO•和CH3C(O)OO•)的两电子转移具有更低的自由能。此外,热力学上不利的CH3COO•解吸进一步促进了Fe(IV)的生成。PAA/MIL-101(Fe)-NH2体系有效降解SMX (kapp= 121.2−287.2 M−1s−1)和其他ec (kapp= 40−432 M−1s−1),水基质组分干扰最小,可重复使用。该研究表明MIL-101(Fe)-NH2是一种强大的PAA活化催化剂,为选择性生成Fe(IV)降解ec提供了一种新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Amino-Functionalized MIL-101(Fe)-NH2 as Efficient Peracetic Acid Activator for Selective Contaminant Degradation: Unraveling the Role of Electron-Donating Ligands in Fe(IV) Generation
Peracetic acid-based advanced oxidation processes (PAA–AOPs), which generate various reactive radicals, have garnered substantial attention for degradation emerging contaminants (ECs). However, nonselective radical-based PAA–AOPs often suffer from interference by water matrix components, causing low contaminants removal efficiency. This study explores the use of amino-(NH2)-functionalized metal–organic frameworks (MIL-101(Fe)-NH2) as heterogeneous catalysts for PAA activation, enabling the generation of high-valent iron- (Fe)–oxo species (Fe(IV)) capable of efficiently degrading ECs (80−100%, within 30 min). The Fe(II) clusters in MIL-101(Fe)-NH2, modulated by electron-donating −NH2 groups, play a pivotal role in Fe(IV) generation. Scavenger and probe experiments confirmed Fe(IV) as the primary reactive species responsible for ECs degradation. Density functional theory calculations demonstrated that the four-electron transfer to generate Fe(IV) has lower free energy than the two-electron transfer to generate organic radicals (e.g., CH3COO• and CH3C(O)OO•). Furthermore, thermodynamically unfavorable CH3COO• desorption further promotes Fe(IV) generation. The PAA/MIL-101(Fe)-NH2 system efficiently degraded SMX (kapp= 121.2−287.2 M−1s−1) and other ECs (kapp= 40−432 M−1s−1) with minimal interference from water matrix components and excellent reusability. This study demonstrates that MIL-101(Fe)-NH2 is a robust catalyst for PAA activation and provides a novel approach for selectively generating Fe(IV) for ECs degradation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


