钌催化氮杂苷苯甲酸酯氧化β消除生成1-氮杂苷
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氮杂环和氮杂环是四元含氮杂环,具有低亲脂性和刚性等独特性质,在药物设计和合成中具有良好的应用前景。然而,它们的合成面临挑战,特别是氮化嘌呤,其可及性有限,阻碍了其应用的探索。我们在此描述的创新方法涉及钌催化氧化消除,利用氮杂啶氮上的羟胺取代基。该方法具有较温和的条件,使22种氮杂啶合成1-氮杂啶的收率可达99%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
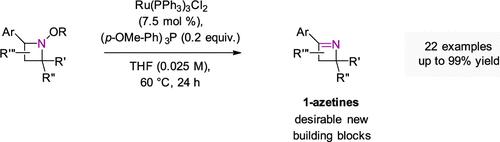
Ruthenium-Catalyzed Formation of 1-Azetines via Oxidative β-Elimination from Azetidine Benzoates
Azetidines and azetines, four-membered nitrogen-containing heterocycles, exhibit unique properties such as low lipophilicity and rigidity, making them promising scaffolds in pharmaceutical design and as synthetic building blocks. However, challenges in their synthesis, particularly azetines, have limited accessibility, hindering the exploration of their applications. Our innovative approach described herein involves a ruthenium-catalyzed oxidative elimination, utilizing a hydroxylamine substituent on the azetidine nitrogen. This method offers milder conditions, enabling the synthesis of 1-azetines from 22 azetidines in yields up to 99%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


