在个性化免疫治疗中增强肿瘤抗原递送的没食子酰化toll样受体7/8激动剂纳米疫苗
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
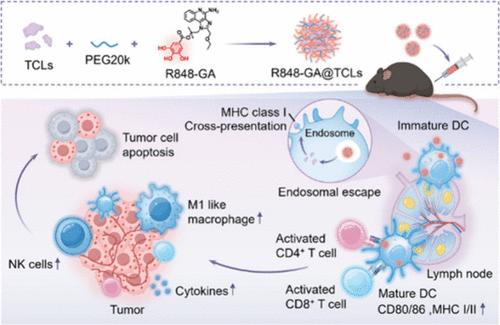
癌症疫苗是癌症免疫治疗的关键技术,已显示出巨大的治疗潜力。然而,基于肿瘤细胞裂解物(TCLs)的传统疫苗在早期临床试验中表现出令人失望的结果,因为免疫原性低,体内不稳定,不能与佐剂共同递送。为了解决这些问题,我们开发了一种纳米颗粒疫苗R848-GA@TCLs,通过用没食子酸修饰toll样受体7/8 (TLR7/8)激动剂R848。这种纳米疫苗利用没氯丙基部分的“捕获”能力来装载tcl和R848,形成稳定的纳米颗粒。R848-GA@TCLs有效靶向淋巴结,使TCL积累增加10倍,并使树突状细胞(DCs)内抗原和佐剂同步释放。我们的研究结果表明,R848-GA@TCLs增加了肿瘤抗原的交叉呈递,促进了促炎细胞因子的产生,并激活了dc,导致效应T细胞、自然杀伤细胞(NK)细胞和M1巨噬细胞的显著增加。这种强烈的免疫反应导致了强大的抗肿瘤作用,R848-GA@TCLs通过显著抑制肿瘤生长和转移在多种肿瘤模型中显示出疗效。总之,R848-GA@TCLs代表了一种个性化的癌症疫苗,能够共同递送tcl和佐剂,引发强大的抗肿瘤免疫反应,具有巨大的临床应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Galloylated Toll-Like Receptor 7/8 Agonist Nanovaccine for Enhanced Tumor Antigen Delivery in Personalized Immunotherapy
Cancer vaccines, a critical technology in cancer immunotherapy, have shown great therapeutic potential. However, traditional vaccines based on tumor cell lysates (TCLs) have shown disappointing results in early clinical trials due to low immunogenicity, in vivo instability, and the inability to codeliver with adjuvants. To address these issues, we developed a nanoparticle vaccine, R848-GA@TCLs, by modifying the toll-like receptor 7/8 (TLR7/8) agonist R848 with gallic acid. This nanovaccine leverages the “capturing” ability of the galloyl moiety to coload TCLs and R848, forming stable nanoparticles. R848-GA@TCLs efficiently target lymph nodes, increasing TCL accumulation 10-fold, and enable the synchronized release of antigens and adjuvants within dendritic cells (DCs). Our results show that R848-GA@TCLs increase with respect to the cross-presentation of tumor antigens, promote the production of pro-inflammatory cytokines, and activate DCs, leading to a significant increase in effector T cells, natural killer (NK) cells, and M1 macrophages. This strong immune response resulted in potent antitumor effects, with R848-GA@TCLs demonstrating efficacy in multiple tumor models by significantly inhibiting tumor growth and metastasis. In conclusion, R848-GA@TCLs represent a personalized cancer vaccine capable of codelivering TCLs and adjuvants, eliciting robust antitumor immune responses, and hold great potential for clinical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


