多相酶活性在磷酸钙纳米材料形成中的作用
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
骨中磷酸钙(CaP)的酶促形成是生物体如何利用酶精确调节生物矿物成核和生长的一个主要例子,为仿生纳米材料的设计提供了灵感。本研究利用单分子酶学和低温透射电镜技术研究了缓冲溶液中酶促性CaP的形成机制,探讨了组织非特异性碱性磷酸酶(TNALP)活性与CaP形成途径的关系。我们表征了非均相TNALP活性的动力学,它由单活性和双活性酶分子在酶催化CaP形成过程中组成,并确定了两种不同的形成途径,产生无定形磷酸钙纳米颗粒和八磷酸钙纳米颗粒。这些结果显示了多相酶活性与CaP形成机制之间复杂而潜在的强大关系,为仿生纳米材料的设计提供了一个新的空间和时间控制水平。本文章由计算机程序翻译,如有差异,请以英文原文为准。
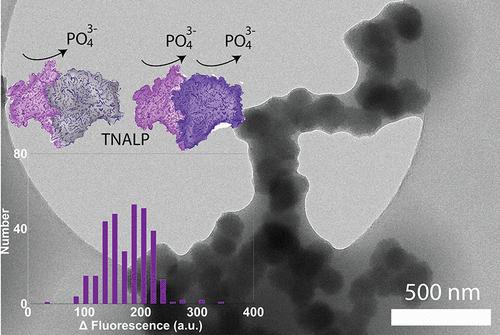
Role of Heterogeneous Enzyme Activity in the Formation of Calcium Phosphate Nanomaterials
The enzymatic formation of calcium phosphate (CaP) in bone is a prime example of how living organisms utilize enzymes to precisely regulate biomineral nucleation and growth, serving as inspiration for the design of biomimetic nanomaterials. In this study, we investigated the formation mechanisms of enzymatic CaP in buffered solutions using single molecule enzymology and cryo-transmission electron microscopy to probe the relationship between tissue nonspecific alkaline phosphatase (TNALP) activity and CaP formation pathways. We characterized the dynamics of heterogeneous TNALP activity, which consists of singly and doubly active enzyme molecules during enzymatic CaP formation and identified two distinct formation pathways that produce amorphous calcium phosphate nanoparticles and octacalcium phosphate nanoparticles. These results show the complex and potentially powerful relationship between heterogeneous enzyme activity and CaP formation mechanisms, offering a new level of control in space and time for the design of biomimetic nanomaterials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


