神经肽的不对称全合成与结构重配
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报道了(+)和(−)两种形式的光学活性神经酮的不对称全合成,这是一种最初于2022年从彩色Nervilia concolor中分离出来的天然产物。从市售间苯二酚开始,光纯神经酮通过10步合成,采用正交定向策略和关键的后期Lewis酸介导的高度非对映选择性还原相结合。在圆二色性(CD)光谱中观察到的差异促使神经蛋白的绝对构型从(3S, 8R)重新分配到(3R, 8S)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
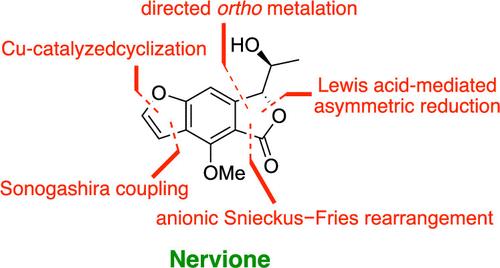
Asymmetric Total Synthesis and Structural Reassignment of Nervione
We report the asymmetric total synthesis of optically active nervione in both (+) and (−) forms, a natural product initially isolated from Nervilia concolor in 2022. Beginning with commercially available resorcinol, optically pure nervione was synthesized in 10 steps, employing a combination of ortho-directed strategies and a crucial late-stage, Lewis acid-mediated, highly diastereoselective reduction. Discrepancies observed in circular dichroism (CD) spectra prompted the reassignment of nervione’s absolute configuration from (3S, 8R) to (3R, 8S).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


