dabco催化噻唑烷-2-硫酮的合成:系统发展和机理研究
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以二硫化碳和α-叔丙胺为原料,采用碱基催化的方法构建了含季碳中心的1,3-噻唑烷-2-硫酮支架。该反应在低催化剂负荷、常温和无溶剂条件下进行。各种α-叔丙胺已被采用,提供了一系列以前未报道的噻唑烷-2-硫酮化合物,并避免在某些情况下通过柱层析纯化。我们还描述了通过KA2耦合- cs2掺入方法合成相同产物的一锅策略。通过详细的实验和计算相结合的方法研究了反应机理和取代基依赖的催化行为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
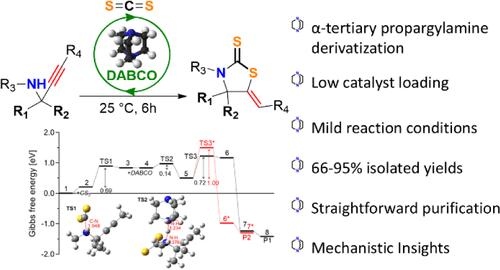
DABCO-Catalyzed Synthesis of Thiazolidine-2-thiones: System Development and Mechanistic Insights
A base-catalyzed protocol is reported for the construction of 1,3-thiazolidine-2-thione scaffolds bearing quaternary carbon centers from carbon disulfide and α-tertiary propargylamines. The reaction proceeds using low catalyst loading, under ambient temperatures, and in the absence of solvent. Various α-tertiary propargylamines have been employed, affording a series of previously unreported thiazolidine-2-thione compounds and avoiding purification via column chromatography in certain cases. We also describe a one-pot strategy for the synthesis of the same products through a KA2 coupling–CS2 incorporation approach. The reaction mechanism and substituent-dependent catalytic behavior were studied through a combination of detailed experimental and computational studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


