二氧化钛-溶液界面竞争吸附的EIS研究
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
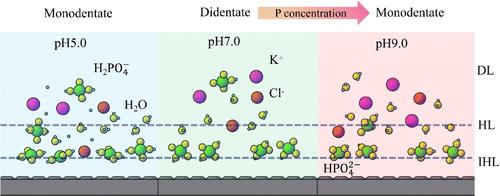
了解离子吸附在电极表面的竞争和结构对电催化反应和腐蚀科学至关重要。在这里,我们展示了如何利用EIS来识别复杂溶液体系中Ti/TiO2电极表面的磷酸盐和氯离子的竞争吸附。通过控制高浓度氯离子(100 mM)和不同pH值(5.0、7.0、9.0)中磷酸离子的浓度,我们清楚地观察到电极表面的吸附物质由最初的氯化物为主向磷酸盐为主转变,以及磷酸盐吸附模式的转变。在阻抗-浓度曲线的低浓度区域,直到大约5 mM P浓度,这种吸附物种的竞争性转变是明显的。此外,与酸性条件相比,中性和碱性条件下的阻抗-浓度曲线在高磷浓度区域显示出类似的开关,表明电极表面的磷酸盐吸附从双齿状向单齿状转变。该研究为后续腐蚀科学中前驱体吸附行为的检测提供了一种方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
EIS Study on Competitive Adsorption at the TiO2–Solution Interface
Understanding the competition and configuration of ionic adsorption on the electrode surface is crucial for electrocatalytic reactions and corrosion science. Here, we demonstrate how EIS can be employed to identify the competitive adsorption of phosphate and chloride ions on the surface of a Ti/TiO2 electrode in a complex solution system. By controlling the concentration of phosphate ions in a high-concentration chloride ion (100 mM) and different pH (5.0, 7.0, and 9.0), we clearly observe a transition in the adsorbed species from initially chloride-dominated to phosphate-dominated on the electrode surface and the transformation of the phosphate adsorption mode. This competitive transition of adsorption species is evident in the low-concentration region of the impedance–concentration curves up to approximately 5 mM P concentration. Furthermore, the impedance–concentration curves under neutral and alkaline conditions show an additional similar switching in the high P concentration region compared with acidic conditions, indicating a shift from bidentate to monodentate phosphate adsorption on the electrode surface. This study provides a method for detecting the adsorption behavior of precursors in subsequent corrosion science.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


