pH和盐度对荚膜红杆菌YO3光发酵产氢和聚羟基丁酸的影响
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究研究了荚膜红杆菌(Rhodobacter capsulatus) YO3(一种摄取氢化酶缺陷(hup−)突变体)对聚羟基丁酸盐(PHB)和H2的产生。研究了生物反应器培养基的pH和盐度作为提高PHB和H2产量的潜在因素。初始控制pH值为7.0 ~ 8.5,盐度为1500 ~ 4000 mgNa+/L。初始试验结果表明,在pH恒定为7.7的条件下,提高培养基的盐度可略微提高PHB的最大产量。在1500 mgNa+/L条件下,pH为8.5时PHB积累量最高(57.5±0.2%),但不稳定且降解迅速。进一步设置以解开pH和盐度的影响。较高的初始pH和盐度水平阻碍了H2的生产,只有在中等胁迫下PHB才能持续生产。在高pH (>8.0)和Na+ (>3000 mg/L)同时施用时,H2和PHB的产量下降最为显著。研究表明,较低的pH值(7.0 ~ 7.5)和Na+浓度(≈1500 mg/L)有利于两种产物的生产。在较高pH或盐度的中等胁迫下,细胞积累并维持PHB,但在高pH和Na+的联合胁迫下,细胞的生产转向H2。恶劣的条件抑制了这两个过程。对于含盐废水的实际应用,pH控制至关重要,因为pH值在7.0-7.5之间可以减少盐度的不利影响。盐度的逐渐增加可能有助于培养物适应,提高底物对H2或PHB生产的有效利用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
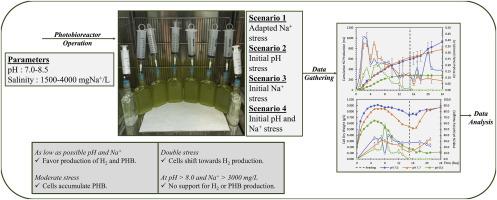
Effect of pH and salinity on photofermentative hydrogen and poly-hydroxybutyric acid production via Rhodobacter capsulatus YO3
The production of poly-hydroxybutyrate (PHB) and H2 via Rhodobacter capsulatus YO3, an uptake hydrogenase deficient (hup−) mutant, was investigated in this study. The pH and salinity of the bioreactor medium were investigated as potential factors to enhance production of PHB and H2. Initial controlled pH and salinity ranges of 7.0–8.5 and 1500–4000 mgNa+/L, respectively, were studied. Initial set demonstrated that under a constant pH of 7.7, increasing the salinity of the medium enhanced maximum PHB production slightly. The highest PHB accumulation (57.5 ± 0.2 %) occurred at pH 8.5 under 1500 mgNa+/L, but was unstable and degraded rapidly. Further sets were run to uncouple the effect of pH and salinity. Higher initial pH and salinity levels hindered H2 productivity, with PHB production sustained only under moderate stress. The most significant drop in both H2 and PHB production occurred with high pH (>8.0) and Na+ (>3000 mg/L) levels applied together. The study suggests that relatively lower pH (7.0–7.5) and Na+ concentration (≈1500 mg/L) favor production of both products. Under moderate stress of either higher pH or salinity, cells accumulate and sustain PHB, but under combined stress of high pH and Na+, production shifts towards H2. Severe conditions inhibit both processes. For practical applications with saline wastewater, pH control is critical, as a pH of 7.0–7.5 can reduce salinity's adverse effects. Gradual increases in salinity may help cultures adapt, enhancing efficient substrate use for H2 or PHB production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


