使用活性氧物种响应脂质体传递系统重编程巨噬细胞表型用于炎症微环境重塑和骨关节炎治疗
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
骨关节炎(OA)的进展与 M1 和 M2 巨噬细胞失衡引起的滑膜炎症密切相关。为了解决这个问题,我们开发了一种对活性氧(ROS)有反应的脂质体,用叶酸配体修饰以靶向 M1 极化的巨噬细胞,并装载了抗炎药物富马酸二甲酯(DMF)。这种基于脂质体的给药系统旨在重编程巨噬细胞表型,重塑关节腔内的炎症微环境,缓解 OA 退化。我们制备的脂质体具有合适的尺寸和负Zeta电位,大小均匀,在水溶液中具有良好的稳定性和出色的生物相容性。实验室测试表明,这些填充了 DMF 的脂质体能显著降低 M1 型巨噬细胞中的高水平 ROS,并通过 Nrf2/HO-1 途径改变巨噬细胞的极化,从而减轻软骨细胞的炎症反应,避免其凋亡。此外,动物实验表明,含有 DMF 的脂质体具有明显的抗炎特性,能显著减轻滑膜炎症,保护损伤的软骨,逆转软骨下骨的变化,并有效减缓前十字韧带横断(ACLT)诱导的小鼠模型中骨关节炎的进展。因此,以 M1 极化巨噬细胞为靶点的 ROS 响应脂质体是治疗 OA 的一种有前景、有价值的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
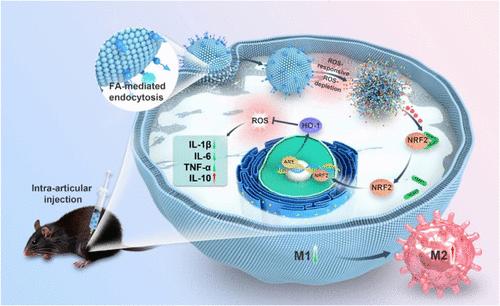
Reprogramming Macrophage Phenotype Using a Reactive Oxygen Species-Responsive Liposome Delivery System for Inflammation Microenvironment Remodeling and Osteoarthritis Treatment
The progression of osteoarthritis (OA) is closely linked to synovial inflammation caused by an imbalance between M1 and M2 macrophages. To tackle this problem, we developed a liposome responsive to reactive oxygen species (ROS), modified with folic acid ligands to target M1-polarized macrophages, and loaded with the anti-inflammatory agent dimethyl fumarate (DMF). This liposome-based drug delivery system was designed to reprogram macrophage phenotype to remodel the inflammatory microenvironment in the joint cavity and alleviate OA degeneration. The liposome we prepared had a suitable size and negative zeta potential, with uniform size, good stability in aqueous solution, and excellent biocompatibility. Laboratory tests showed that these DMF-filled liposomes notably decreased high levels of ROS in M1-type macrophages and shifted macrophage polarization via the Nrf2/HO-1 pathway, which in turn lessened inflammation in chondrocytes and averted their apoptosis. Additionally, animal studies demonstrated that liposomes containing DMF exhibited notable anti-inflammatory properties, significantly reduced synovial inflammation, safeguarded injured cartilage, reversed changes in subchondral bone, and effectively slowed the progression of osteoarthritis in a mouse model induced by anterior cruciate ligament transection (ACLT). Therefore, ROS-responsive liposomes targeting M1-polarized macrophages represent a promising and valuable approach for OA treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


