电子供体-受体激活的超持久氧还原反应的抗芬顿特性
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
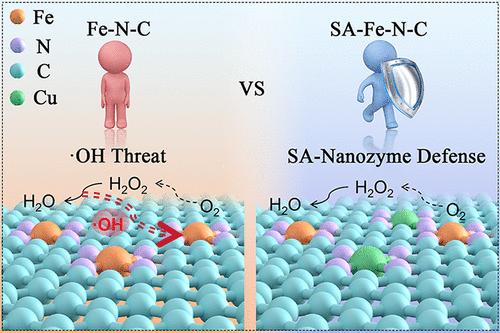
铁氮碳(Fe-N-C)材料被认为是一类不含铂(Pt)的氧还原反应(ORR)催化剂。然而,这些材料的长期稳定性和有效性受到铁原子的溶解和氧化的显著阻碍。Fe-N-C的微观结构工程是提高ORR活性和稳定性的可行途径。本文通过引入单原子Cu,开发了cun5 -单原子纳米酶(SAzyme)辅助Fe-N5催化剂(SA-Fe-N5),以提高Fe-N-C催化剂的ORR性能。电化学评价表明,SA-Fe-N5在碱性溶液中表现出优异的ORR活性,具有与商用Pt/C相似的半波电位和限制扩散的电流密度。基于密度泛函理论的计算表明,单个铜原子可以作为电子供体,提高铁位点的电子密度。该修饰提高了ORR过程中中间体的吸附和解吸能,最终提高了单原子Fe-N5催化剂的ORR性能。此外,Cu位点的引入可以看作是过氧化氢酶单原子纳米酶(CAT-SAzyme),促进了副产物H2O2分解为H2O,从而增强了ORR过程中的抗fenton活性。值得注意的是,作为锌空气电池的阴极催化剂,SA-Fe-N5表现出令人印象深刻的功率密度为217.8 mW cm-2,电流密度为257.3 mA cm-2。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electron Donor–Acceptor Activated Anti-Fenton Property for the Ultradurable Oxygen Reduction Reaction
Iron–nitrogen–carbon (Fe–N–C) materials are recognized as an effective category of catalysts that do not contain platinum (Pt) for the oxygen reduction reaction (ORR). Nonetheless, the long-term stability and effectiveness of these materials are significantly hindered by the dissolution and oxidation of Fe atoms. Microstructural engineering of Fe–N–C is a viable approach to enhancing ORR activity and stability. Herein, CuN5-single-atom nanozymes (SAzyme)-assisted Fe–N5 catalysts (SA–Fe–N5) were developed by introducing single-atom Cu to enhance Fe–N–C catalyst ORR performance. Electrochemical assessments indicated that SA–Fe–N5 exhibited excellent ORR activity in alkaline solutions, with a half-wave potential and a diffusion-limited current density similar to that of commercial Pt/C. Calculations based on density functional theory indicated that a single copper atom can function as an electron donor, enhancing the electron density at the iron sites. This modification improves the adsorption and desorption energies for intermediates involved in the ORR process, ultimately boosting the ORR performance of the single-atom Fe–N5 catalyst. Moreover, the introduction of the Cu site can be regarded as a catalase single-atom nanozyme (CAT-SAzyme), facilitating the decomposition of the byproduct H2O2 to H2O and thereby enhancing the anti-Fenton activity during the ORR process. Notably, as a cathode catalyst in a zinc-air battery, SA–Fe–N5 demonstrated an impressive power density of 217.8 mW cm–2 alongside a current density of 257.3 mA cm–2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


