UV/氯法降解西威因:最佳降解条件、反应中间体和机理
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
威因是一种广泛使用的氨基甲酸酯类杀虫剂,对各种生物和人类都有毒性,尽管其环境降解速度相对较快,但仍持续存在于水循环中。本研究深入研究了紫外/氯处理对西维因的降解,旨在确定最佳降解条件并了解反应机理。考察了氧化剂浓度、溶液pH值、天然有机质(NOM)和碱度对UV/氯法降解西维因的影响。与单独紫外线处理或暗氯化(即无紫外线氯化)相比,紫外线/氯处理显著增强了西维因的降解。在pH 7.0以下(HOCl的pKa为7.5),威因能有效分解;在较高的紫外线剂量下,无论溶液ph如何,西威尼都被完全去除。虽然NOM和碱度通过清除活性物质降低了降解效率,但增加紫外线剂量可以减轻这种影响。此外,恶臭除臭塔内部循环水中残留的NaOCl被有效地重新用于西威因降解,代表了我们实验室研究结果的创新和实际应用。阐明了西威尼的降解途径,揭示了各种中间体通过与羟基自由基(HO·)和反应氯种(RCS)的反应生成。这些发现有助于开发先进的水处理技术,以减轻农业实践产生的持久性有机污染物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
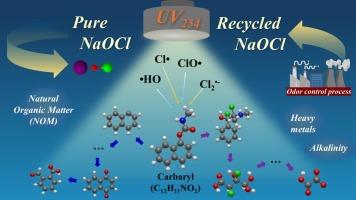
Degradation of carbaryl by UV/chlorine process: Optimal degradation conditions, reaction intermediates, and mechanisms
Carbaryl, a widely used carbamate insecticide, is toxic to various organisms and humans, persisting in the water cycle despite its relatively rapid environmental degradation. This study thoroughly investigates carbaryl degradation using UV/chlorine treatment, aiming to identify optimal degradation conditions and understand reaction mechanisms. The effects of oxidant concentration, solution pH values, natural organic matter (NOM), and alkalinity on carbaryl degradation by UV/chlorine were examined. The UV/chlorine treatment exhibited significantly enhanced carbaryl degradation compared to UV treatment alone or dark chlorination (i.e., chlorination without UV). Carbaryl was effectively decomposed below pH 7.0 (<pKa of HOCl, which is 7.5); at higher UV dosages, carbaryl was completely removed regardless of solution pH. While NOM and alkalinity reduced degradation efficiency by scavenging reactive species, this effect could be mitigated by increasing UV dosages. Furthermore, residual NaOCl in the internal circulating water of an odor deodorization tower was effectively repurposed for carbaryl degradation, representing an innovative, practical application of our laboratory findings. The degradation pathway of carbaryl was also elucidated, revealing the formation of various intermediates through reactions with hydroxyl radicals (HO·) and reaction chlorine species (RCS). These findings contribute to developing advanced water treatment technologies for mitigating persistent organic pollutants derived from agricultural practices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


