空位浓度在锂金属间化合物超原子扩散中的关键作用
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
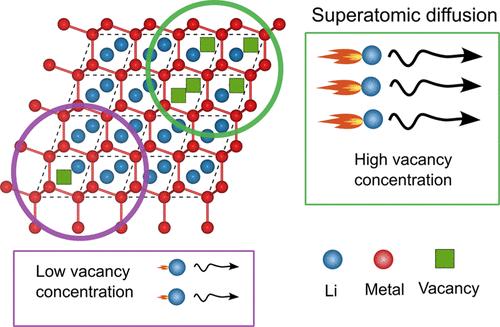
与目前依赖液体电解质的商用电池相比,无阳极固态锂电池有望显著提高能量密度。主要的挑战仍然是在阳极集流器上控制锂金属电镀和剥离过程中的形态演变。元素添加剂可以改变锂合金的镀和剥离行为。许多合金元素与锂形成金属间化合物,锂通过这些金属间化合物的迁移率被认为对形态演化有重要影响。本研究表明,金属间化合物的Li输运系数范围很大,B32 LiAl金属间化合物在室温下的Li示踪剂扩散系数高达10-6 cm2/s,比同构B32 LiZn的扩散系数大8个数量级。这项工作证明了空位浓度在通过金属间化合物控制Li原子迁移率方面的关键作用。虽然LiAl和LiZn中li -空位交换的迁移障碍都非常低,但LiAl中的超原子导电性是由B32 LiAl化合物独特的电子结构引起的,有利于高浓度的空位。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Crucial Role of Vacancy Concentration in Enabling Superatomic Diffusion in Lithium Intermetallics
Anode-free solid-state Li batteries promise significant increases in energy densities compared to current commercial batteries that rely on liquid electrolytes. Major challenges persist in controlling morphological evolution during the plating and stripping of lithium metal at the anode current collector. Elemental additives that alloy with lithium have been found to modify the plating and stripping behavior of lithium. Many alloying elements form intermetallics with lithium and the mobility of Li through these intermetallics is believed to have an important effect on morphological evolution. This study shows that Li transport coefficients through intermetallics span a wide range in values, with the B32 LiAl intermetallic predicted to have a Li tracer diffusion coefficient as high as 10–6 cm2/s at room temperature, which is 8 orders of magnitude larger than that of isostructural B32 LiZn. This work demonstrates the crucial role of vacancy concentration in controlling the mobility of Li atoms through intermetallics. While the migration barriers for Li-vacancy exchanges in both LiAl and LiZn are remarkably low, the superatomic conductivity in LiAl is shown to arise from the unique electronic structure of the B32 LiAl compound, which favors high concentrations of vacancies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


