天然产物中吲哚和吲哚啉骨架合成的新进展
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
吲哚生物碱是自然界中发现的最重要的天然产物之一,特别是在各种植物中。这些化合物具有至少有一个氮原子的紧凑的多环体系。这些生物碱中有几种具有生物活性,为开发新药带来了希望。它们的生物合成涉及色氨酸作为氨基酸前体,因为吲哚或吲哚部分是这些天然产物的主要杂环。然而,在合成这种复杂结构的过程中,化学家们已经开发出几种不同的策略,以一种非自然的方式快速生产出这种关键的杂环。本文综述了近年来用于制备这些重要生物碱的吲哚和吲哚核的全合成方法。允许快速形成这种杂环的新颖和古老的方法被描述为自然母亲设计的这些迷人结构的全面合成的关键步骤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
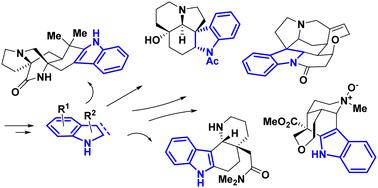
Recent strategy for the synthesis of indole and indoline skeletons in natural products
Indole alkaloids are one of the most important classes of natural products found in nature, particularly in a wide variety of plants. These compounds have compact polycyclic systems with at least one nitrogen atom. Several of these alkaloids are bioactive and have raised hopes for the development of new drugs. Their biosynthesis involves tryptophan as an amino acid precursor, since the indole or indoline moiety is the main heterocycle of these natural products. However, in their quest to synthesize such complex architectures, chemists have developed several different strategies to produce this key heterocycle quickly and in an unnatural way. This review focuses on the recent total synthesis methods used to prepare the indole and indoline core of these important alkaloids. Novel and older methods that allow the rapid formation of this heterocycle are described as key steps in the total synthesis of these fascinating structures designed by Mother Nature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


