磁铁矿化学计量学(Fe(II)/Fe(III))控制三价铬表面形态
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
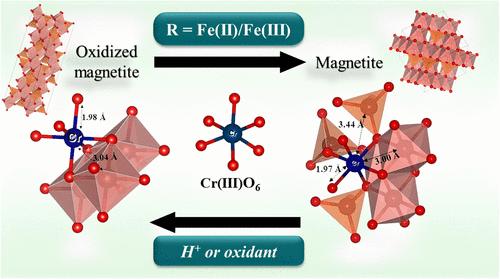
虽然通过在磁铁矿(Fe3O4)表面还原为Cr(III)来消除最有毒形式的铬(Cr(VI))已经被广泛记录,但阐明Cr(III)吸附到磁铁矿的确切机制却引起了较少的关注。事实上,磁铁矿化学计量(R = Fe(II)/Fe(III))在cr -磁铁矿相互作用研究中很少被控制或监测,尽管它被证明不仅影响氧化还原转化,还影响几种污染物的吸附机制。本研究研究了20 μM (~ 1 mg L-1) Cr(III)与10 nm磁铁矿的相互作用,其化学计量被仔细定义为(0.1≤R≤0.5),并在10 mM NaCl厌氧条件下保存。x射线吸收光谱显示,在酸性条件下,氧化磁铁矿(R0.1)或化学测量磁铁矿(R0.5)表面形成了一个三叉状的三核内球表面配合物,其中H+促进溶解产生了Fe(II)贫表面。当磁铁矿化学比增加时,Cr的表面形态有利于形成[Fe2+Crx3+Fe3+ 1-x]OhFeTd3+ o4样固溶体,其中Cr(III)部分取代了八面体位置上的Fe(III)。本研究揭示了pH和磁铁矿化学计量对Cr(III)吸附机理的共同影响,表明Cr(III)-(氢氧化物)氧化物的沉淀不一定是Cr(III)从溶液中消除的驱动过程。这些结果将有助于预测铬的命运和迁移,以及开发磁铁矿基铬修复工艺。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Magnetite Stoichiometry (Fe(II)/Fe(III)) Controls on Trivalent Chromium Surface Speciation
While the elimination of the most toxic form of chromium (Cr(VI)) by its reduction to Cr(III) at the magnetite (Fe3O4) surface has widely been documented, elucidating the exact mechanism involved in Cr(III) sorption to magnetite has attracted less attention. Indeed, magnetite stoichiometry (R = Fe(II)/Fe(III)) is rarely controlled or monitored in Cr-magnetite interaction studies, although it was shown to affect not only redox transformation but also adsorption mechanisms of several contaminants. This study examined the interaction of 20 μM (∼1 mg L–1) Cr(III) with 10 nm magnetites, whose stoichiometries were carefully defined (0.1 ≤ R ≤ 0.5) and preserved under anaerobic conditions in 10 mM NaCl. X-ray absorption spectroscopy showed the formation of a tridentate trinuclear inner-sphere surface complex, but it occurred only on oxidized magnetite (R0.1) or on stoichiometric magnetite (R0.5) under acidic conditions, where H+-promoted dissolution generated an Fe(II)-depleted surface. When magnetite stoichiometry increased, Cr surface speciation evolved in favor of a [Fe2+Crx3+Fe3+ 1–x]OhFeTd3+O4-like solid solution in which Cr(III) partially substitutes Fe(III) in octahedral sites. This study reveals the joint effects of pH and magnetite stoichiometry on the Cr(III) sorption mechanism, demonstrating that Cr(III)-(hydr)oxide precipitation is not necessarily the driving process of Cr(III) elimination from solutions. These results will help predict the fate and transport of chromium as well as develop magnetite-based chromium remediation processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


