亚临界水热条件下PFBS降解机理的理论与实验研究
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
全氟和多氟烷基物质(PFAS)以其强大的C-F键而闻名,这使它们在环境中具有高度持久性和抗降解性。全氟辛烷磺酸(PFBS)是全氟辛酸(PFOA)和全氟辛烷磺酸(PFOS)等长链全氟辛烷磺酸被广泛用作替代品的一种短链全氟辛烷磺酸,由于其环境持久性和毒性而引起人们的关注。尽管PFBS的生物蓄积潜力低于长链污染物,但它仍然是一种重要的污染物,有关其降解机制的数据有限。为了解决这个问题,本研究研究了PFBS在亚临界水热液条件下的降解。测试了各种碱性物质和添加剂,并采用液相和气相色谱-质谱法对降解产物进行了分析。结果表明,在325℃条件下,2 M NaOH对PFBS的去除率为~99.4%,降解途径从S-C键断裂开始,然后是C- f键断裂,形成较小的氟化化合物,包括三氟乙酸。密度泛函理论(DFT)计算提供了对降解机制的详细见解,确定了氢氧根离子对磺酸基的攻击是第一步,并阐明了后续反应的三种不同途径。这项研究为PFBS的降解机制提供了关键的见解,强调了碱性碱和添加剂的协同作用。研究结果支持了亚临界水热法去除PFAS的优化,提供了一种可扩展且环境可持续的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
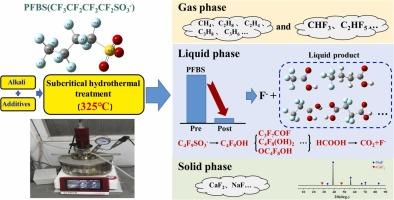
Theoretical and experimental insights into the degradation mechanism of PFBS under subcritical hydrothermal conditions
Per- and polyfluoroalkyl substances (PFAS) are known for their strong C-F bonds, which make them highly persistent in the environment and resistant to degradation. Among PFAS, perfluorobutane sulfonate (PFBS), a short-chain PFSA widely used as a replacement for long-chain PFAS like perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), has garnered attention due to its environmental persistence and toxicity. Although PFBS has lower bioaccumulation potential than its long-chain counterparts, it remains a significant pollutant with limited data available on its degradation mechanisms. To address this, the present study investigates PFBS degradation under subcritical water hydrothermal conditions. Various alkaline substances and additives were tested, and degradation products were analyzed by liquid and gas chromatography-mass spectrometry. Results show that 2 M NaOH at 325°C achieved an ∼99.4 % PFBS removal rate, and the degradation pathway began with the breakdown of the S-C bond, followed by C-F bond cleavage, resulting in the formation of smaller fluorinated compounds, including trifluoroacetic acid. Density functional theory (DFT) calculations provided detailed insights into the degradation mechanism, identifying hydroxide ion attack on the sulfonic acid group as the initial step and elucidating three distinct pathways for subsequent reactions. This study provides key insights into PFBS degradation mechanisms, emphasizing the synergistic effect of alkaline bases and additives. The findings support the optimization of subcritical hydrothermal treatment for PFAS removal, providing a scalable and environmentally sustainable solution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


