电池及其他领域的溶剂共插层反应
IF 55.8
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
溶剂共插层是指在电池充放电过程中,离子和溶剂共同插层到层状电极材料中的过程。它通常会导致电极的快速降解,但新的发现表明,它可以是高度可逆的,持续几千个循环。溶剂共插层有两个重要的特点:(1)由于溶剂化壳的剥离被消除,电荷转移阻力被最小化;(2)溶剂成为电极反应的一部分,这一事实为设计电极材料提供了另一种手段。溶剂共插层的概念在化学上是非常多样化的,因为一个电极材料可以容纳不同类型和数量的溶剂和离子。很可能存在许多未被发现的电极材料、溶剂和离子的组合,能够进行溶剂共插反应,为新材料提供了很大程度上未被探索的化学空间。共插层可以扩展晶格(1nm),使自由溶剂存在于结构中,形成层状的“多孔”材料。这表明该概念具有更广泛的影响,并涉及到其他研究领域,如超级电容器、层状纳米结构和纳米催化。本文综述了溶剂共插层反应的概念、研究现状、表征方法、优点、局限性以及今后的研究方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
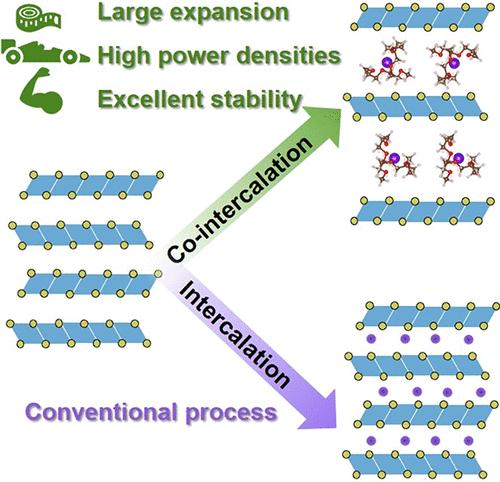
Solvent Co-Intercalation Reactions for Batteries and Beyond
Solvent co-intercalation is a process in which ions and solvents jointly intercalate into a layered electrode material during battery charging/discharging. It typically leads to rapid electrode degradation, but new findings show that it can be highly reversible, lasting several thousand cycles. Solvent co-intercalation has two important characteristics: (1) the charge transfer resistance is minimized as stripping of the solvation shell is eliminated and (2) the fact that solvents become part of the electrode reaction provides another means of designing electrode materials. The concept of solvent co-intercalation is chemically very diverse, as a single electrode material can host different types and numbers of solvents and ions. It is likely that many undiscovered combinations of electrode materials, solvents, and ions capable of solvent co-intercalation reactions exist, offering a largely unexplored chemical space for new materials. Co-intercalation can expand the crystal lattice (>1 nm) to the extent that free solvents are present in the structure, forming a layered, “porous” material. This indicates that the concept has a much broader impact and relates to other research fields such as supercapacitors, layered nanostructures, and nanocatalysis. This Review covers the concept and current understanding of solvent co-intercalation reactions, characterization methods, advantages, limitations, and future research directions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Reviews
化学-化学综合
CiteScore
106.00
自引率
1.10%
发文量
278
审稿时长
4.3 months
期刊介绍:
Chemical Reviews is a highly regarded and highest-ranked journal covering the general topic of chemistry. Its mission is to provide comprehensive, authoritative, critical, and readable reviews of important recent research in organic, inorganic, physical, analytical, theoretical, and biological chemistry.
Since 1985, Chemical Reviews has also published periodic thematic issues that focus on a single theme or direction of emerging research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


