合成核肽和核苷酸的动力学捕获自组装
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
大自然经常使用热力学上不利的动态自组装来执行细胞功能。一个核肽和ATP形成自组装水凝胶。肽和AMP不能形成水凝胶。值得注意的是,当ATP被ATP酶原位水解成AMP时,肽-AMP组合物仍然是自组装的水凝胶,表现出不同寻常的动力学捕获自组装本文章由计算机程序翻译,如有差异,请以英文原文为准。
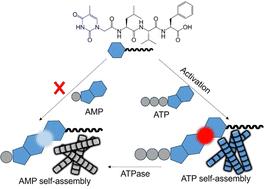
Kinetically trapped self-assembly in synthetic nucleopeptides and nucleotides†
Nature uses thermodynamically unfavourable dynamic self-assembly to execute cellular functions frequently. A nucleopeptide and ATP formed a self-assembled hydrogel. The peptide and AMP were unable to form a hydrogel. Notably, when ATP was hydrolyzed to AMP in situ by an ATPase enzyme, the peptide–AMP composition remained a self-assembled hydrogel, showing unusual kinetically trapped self-assembly.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


